Assessment of Favipiravir and Remdesivir in Combination for SARS-CoV-2 Infection in Syrian Golden Hamsters
- PMID: 39772148
- PMCID: PMC11680105
- DOI: 10.3390/v16121838
Assessment of Favipiravir and Remdesivir in Combination for SARS-CoV-2 Infection in Syrian Golden Hamsters
Abstract
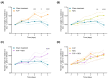
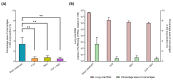
Favipiravir (FVP) and remdesivir (RDV) have demonstrable antiviral activity against SARS-CoV-2. Here, the efficacy of FVP, RDV, and FVP with RDV (FVP + RDV) in combination was assessed in Syrian golden hamsters challenged with SARS-CoV- 2 (B.1.1.7) following intraperitoneal administration. At day 4 post infection, viral RNA and viral antigen expression were significantly lower in lungs for all three treatment groups compared to the sham treatment. Similarly, viral titres in the lungs were lower in all treatment groups compared to the sham treatment. The FVP + RDV combination was the only treatment group where viral RNA in nasal turbinate and lung, virus titres in lung, and viral antigen expression (lung) were all lower than those for the sham treatment group. Moreover, lower viral titre values were observed in the FVP + RDV group compared to other treatment groups, albeit only significantly lower in comparison to those in the RDV-only-treated group. Further assessment of the potential utility of FVP in combination with RDV may be warranted. Future studies should also consider whether the combination of these two drugs may reduce the speed at which drug resistance mutations are selected.
Keywords: SARS-CoV-2; combination therapy; favipiravir; remdesivir.
Conflict of interest statement
MN is currently employed by the Wellcome Trust and is a co-inventor of patents relating to drug delivery. AO is a Director of Tandem Nano Ltd. and co-inventor of patents relating to drug delivery. AO has received research funding from ViiV Healthcare and Gilead unrelated to COVID-19, and consultancy from Gilead and Assembly Biosciences also not related to COVID-19. JPS has received research funding from ENA respiratory, and consultancy from Byotrol Technologies, not related to the current paper. No other conflicts are declared by the authors.
Figures

Similar articles
-
Chemoprophylactic Assessment of Combined Intranasal SARS-CoV-2 Polymerase and Exonuclease Inhibition in Syrian Golden Hamsters.Viruses. 2023 Oct 27;15(11):2161. doi: 10.3390/v15112161. Viruses. 2023. PMID: 38005839 Free PMC article.
-
Therapeutic dilemmas in addressing SARS-CoV-2 infection: Favipiravir versus Remdesivir.Biomed Pharmacother. 2022 Mar;147:112700. doi: 10.1016/j.biopha.2022.112700. Epub 2022 Feb 4. Biomed Pharmacother. 2022. PMID: 35131656 Free PMC article. Review.
-
The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model.EBioMedicine. 2021 Oct;72:103595. doi: 10.1016/j.ebiom.2021.103595. Epub 2021 Sep 24. EBioMedicine. 2021. PMID: 34571361 Free PMC article.
-
Beneficial effect of combinational methylprednisolone and remdesivir in hamster model of SARS-CoV-2 infection.Emerg Microbes Infect. 2021 Dec;10(1):291-304. doi: 10.1080/22221751.2021.1885998. Emerg Microbes Infect. 2021. PMID: 33538646 Free PMC article.
-
Tackling COVID-19 Using Remdesivir and Favipiravir as Therapeutic Options.Chembiochem. 2021 Mar 16;22(6):939-948. doi: 10.1002/cbic.202000595. Epub 2020 Nov 11. Chembiochem. 2021. PMID: 33031623 Free PMC article. Review.
References
-
- Dhama K., Nainu F., Frediansyah A., Yatoo M.I., Mohapatra R.K., Chakraborty S., Zhou H., Islam M.R., Mamada S.S., Kusuma H.I., et al. Global emerging Omicron variant of SARS-CoV-2: Impacts, challenges and strategies. J. Infect. Public Health. 2023;16:4–14. doi: 10.1016/j.jiph.2022.11.024. - DOI - PMC - PubMed
-
- Shannon A., Selisko B., Le N.T., Huchting J., Touret F., Piorkowski G., Fattorini V., Ferron F., Decroly E., Meier C., et al. Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis. Nat. Commun. 2020;11:4682. doi: 10.1038/s41467-020-18463-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AI134091/GF/NIH HHS/United States
- R24AI118397/GF/NIH HHS/United States
- LONGEVITY (2020-38-LONGEVITY)/Unitaid
- EP/R024804/1; EP/S012265/1/EPSRC
- MR/W005611/1/MRC
- MR/R010145/1/MRC
- BB/R00904X/1/BBSRC
- BB/R018863/1/BBSRC
- BB/N022505/1/BBSRC
- TS/V012967/1/Innovate UK
- 871029/European Union's Horizon 2020 research and innovation programme
- IZSEZ0_213289/SNSF_/Swiss National Science Foundation/Switzerland
- MR/836 S00467X/1/MRC
- SIPF 20197/UK Research and Innovation Strength in Places Fund
LinkOut - more resources
Full Text Sources
Miscellaneous

