Predictive Efficacy of Dual Therapies Combining Integrase Strand Transfer Inhibitors with Second-Generation Non-Nucleoside Reverse Transcriptase Inhibitors Following HIV-1 Treatment Failure in Cameroon: Implications for the Use of a Long-Acting Therapeutic Strategy in Low- and Middle-Income Countries
- PMID: 39772163
- PMCID: PMC11680099
- DOI: 10.3390/v16121853
Predictive Efficacy of Dual Therapies Combining Integrase Strand Transfer Inhibitors with Second-Generation Non-Nucleoside Reverse Transcriptase Inhibitors Following HIV-1 Treatment Failure in Cameroon: Implications for the Use of a Long-Acting Therapeutic Strategy in Low- and Middle-Income Countries
Abstract
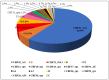
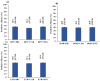
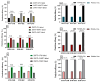
Dual therapies (DT) combining integrase strand transfer inhibitors (INSTIs) with second-generation non-nucleoside reverse transcriptase inhibitors (2nd-Gen-NNRTIs) offer new possibilities for HIV treatment to improve adherence. However, drug resistance associated mutations (RAMs) to prior antiretrovirals may jeopardize the efficacy of DT. We herein describe the predicted efficacy of DT combining INSTIs + 2nd-Gen-NNRTI following treatment failure among Cameroonian patients. We genotyped the HIV-1 pol gene using Sanger sequencing and assessed acquired RAMs to NNRTIs and INSTIs in patients failing treatment from March 2019 to December 2023. Drug susceptibility was interpreted using Stanford HIVdb v9.5, and statistical analyses were performed using SPSS v22. Of 130 successfully genotyped participants (median age (IQR): 38 (27-46) years; 59.2% female), 92.3% had RAMs to NNRTIs and 1.5% to INSTIs. Prevailing RAMs were Y181C (32.3%) among NNRTIs and R263K (0.7%) among INSTIs. Among 2nd-Gen-NNRTIs, etravirine, doravirine and rilpivirine had 43.85%, 41.54% and 38.46% genotypic sensitivity, respectively. Among INSTIs, we found 97.69% efficacy for dolutegravir/bictegravir, 96.15% for cabotegravir and 92.31% for elvitegravir/raltegravir. The overall predictive efficacy of DT was lower among participants who failed 1st-Gen-NNRTI (p < 0.001); with etravirine + dolutegravir/bictegravir combination showing the highest score (43.8%). Conclusively, DT combining INSTIs + 2nd-Gen-NNRTIs might be suboptimal in the context of previous ART failure, especially with NNRTI-based treatment in low- and middle-income countries. The general data clearly indicate that without resistance testing, it is nearly impossible to use long-acting dual therapies in previously failing patients.
Keywords: Cameroon; HIV-1; dual therapies; integrase strand transfer inhibitor; non-nucleosides reverse transcriptase inhibitors; predictive efficacy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- UNAIDS Global HIV & AIDS Statistics—Fact Sheet. [(accessed on 18 May 2024)]. Available online: https://www.unaids.org/en/resources/fact-sheet.
-
- [Comité National de Lutte Contre Le SIDA—CNLS] [Cnls.Cm] [(accessed on 17 May 2024)]. Available online: https://cnls.cm/site/fr/home-page-1.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

