Heterogeneous Ribonucleoprotein K Is a Host Regulatory Factor of Chikungunya Virus Replication in Astrocytes
- PMID: 39772225
- PMCID: PMC11680317
- DOI: 10.3390/v16121918
Heterogeneous Ribonucleoprotein K Is a Host Regulatory Factor of Chikungunya Virus Replication in Astrocytes
Abstract
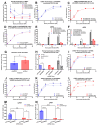
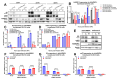
Chikungunya virus (CHIKV) is an emerging, mosquito-borne arthritic alphavirus increasingly associated with severe neurological sequelae and long-term morbidity. However, there is limited understanding of the crucial host components involved in CHIKV replicase assembly complex formation, and thus virus replication and virulence-determining factors, within the central nervous system (CNS). Furthermore, the majority of CHIKV CNS studies focus on neuronal infection, even though astrocytes represent the main cerebral target. Heterogeneous ribonucleoprotein K (hnRNP K), an RNA-binding protein involved in RNA splicing, trafficking, and translation, is a regulatory component of alphavirus replicase assembly complexes, but has yet to be thoroughly studied in the context of CHIKV infection. We identified the hnRNP K CHIKV viral RNA (vRNA) binding site via sequence alignment and performed site-directed mutagenesis to generate a mutant, ΔhnRNPK-BS1, with disrupted hnRNPK-vRNA binding, as verified through RNA coimmunoprecipitation and RT-qPCR. CHIKV ΔhnRNPK-BS1 demonstrated hampered replication in both NSC-34 neuronal and C8-D1A astrocytic cultures. In astrocytes, disruption of the hnRNPK-vRNA interaction curtailed viral RNA transcription and shut down subgenomic RNA translation. Our study demonstrates that hnRNP K serves as a crucial RNA-binding host factor that regulates CHIKV replication through the modulation of subgenomic RNA translation.
Keywords: RNA-binding protein; alphavirus; astrocytes; chikungunya virus; heterogeneous ribonucleoprotein K; host–virus interactions; neurons; neurovirulence; subgenomic RNA translation; viral replication.
Conflict of interest statement
Diane E. Griffin is a member of the advisory boards for GlaxoSmithKline, Academia Sinica, and the University of Vermont and has also received funding from Gilead, MeVox, Merck, and the US National Institutes of Health.
Figures



References
-
- Silva M.M.O., Tauro L.B., Kikuti M., Anjos R.O., Santos V.C., Goncalves T.S.F., Paploski I.A.D., Moreira P.S.S., Nascimento L.C.J., Campos G.S., et al. Concomitant Transmission of Dengue, Chikungunya, and Zika Viruses in Brazil: Clinical and Epidemiological Findings From Surveillance for Acute Febrile Illness. Clin. Infect. Dis. 2019;69:1353–1359. doi: 10.1093/cid/ciy1083. - DOI - PMC - PubMed
-
- Mehta R., Soares C.N., Medialdea-Carrera R., Ellul M., da Silva M.T.T., Rosala-Hallas A., Jardim M.R., Burnside G., Pamplona L., Bhojak M., et al. The spectrum of neurological disease associated with Zika and chikungunya viruses in adults in Rio de Janeiro, Brazil: A case series. PLoS Negl. Trop. Dis. 2018;12:e0006212. doi: 10.1371/journal.pntd.0006212. - DOI - PMC - PubMed
-
- Balavoine S., Pircher M., Hoen B., Herrmann-Storck C., Najioullah F., Madeux B., Signate A., Valentino R., Lannuzel A., Saint Louis M., et al. Guillain-Barre Syndrome and Chikungunya: Description of All Cases Diagnosed during the 2014 Outbreak in the French West Indies. Am. J. Trop. Med. Hyg. 2017;97:356–360. doi: 10.4269/ajtmh.15-0753. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

