Development and Evaluation of a Newcastle Disease Virus-like Particle Vaccine Expressing SARS-CoV-2 Spike Protein with Protease-Resistant and Stability-Enhanced Modifications
- PMID: 39772238
- PMCID: PMC11680274
- DOI: 10.3390/v16121932
Development and Evaluation of a Newcastle Disease Virus-like Particle Vaccine Expressing SARS-CoV-2 Spike Protein with Protease-Resistant and Stability-Enhanced Modifications
Abstract
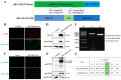
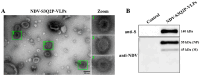
The ongoing global health crisis caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) necessitates the continuous development of innovative vaccine strategies, especially in light of emerging viral variants that could undermine the effectiveness of existing vaccines. In this study, we developed a recombinant virus-like particle (VLP) vaccine based on the Newcastle Disease Virus (NDV) platform, displaying a stabilized prefusion form of the SARS-CoV-2 spike (S) protein. This engineered S protein includes two proline substitutions (K986P, V987P) and a mutation at the cleavage site (RRAR to QQAQ), aimed at enhancing both its stability and immunogenicity. Using a prime-boost regimen, we administered NDV-VLP-S-3Q2P intramuscularly at different doses (2, 10, and 20 µg) to BALB/c mice. Robust humoral responses were observed, with high titers of S-protein-specific IgG and neutralizing antibodies against SARS-CoV-2 pseudovirus, reaching titers of 1:2200-1:2560 post-boost. The vaccine also induced balanced Th1/Th2 immune responses, evidenced by significant upregulation of cytokines (IFN-γ, IL-2, and IL-4) and S-protein-specific IgG1 and IgG2a. Furthermore, strong activation of CD4+ and CD8+ T cells in the spleen and lungs confirmed the vaccine's ability to promote cellular immunity. These findings demonstrate that NDV-S3Q2P-VLP is a potent immunogen capable of eliciting robust humoral and cellular immune responses, highlighting its potential as a promising candidate for further clinical development in combating COVID-19.
Keywords: NDV; SARS-CoV-2; VLP; immune response; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Construction and immunogenicity of SARS-CoV-2 virus-like particle expressed by recombinant baculovirus BacMam.Microbiol Spectr. 2024 Aug 6;12(8):e0095924. doi: 10.1128/spectrum.00959-24. Epub 2024 Jun 25. Microbiol Spectr. 2024. PMID: 38916311 Free PMC article.
-
Intranasal SARS-CoV-2 Omicron variant vaccines elicit humoral and cellular mucosal immunity in female mice.EBioMedicine. 2024 Jul;105:105185. doi: 10.1016/j.ebiom.2024.105185. Epub 2024 Jun 7. EBioMedicine. 2024. PMID: 38848648 Free PMC article.
-
Safety and Immunogenicity Analysis of a Newcastle Disease Virus (NDV-HXP-S) Expressing the Spike Protein of SARS-CoV-2 in Sprague Dawley Rats.Front Immunol. 2021 Nov 18;12:791764. doi: 10.3389/fimmu.2021.791764. eCollection 2021. Front Immunol. 2021. PMID: 34868082 Free PMC article.
-
Addressing SARS-CoV-2 evolution: neutralization of emerging variants of concern by the AVX/COVID-12 'Patria' vaccine based on HexaPro-S ancestral Wuhan spike or its updated BA.2.75.2 version.Front Immunol. 2025 May 19;16:1565934. doi: 10.3389/fimmu.2025.1565934. eCollection 2025. Front Immunol. 2025. PMID: 40458417 Free PMC article.
-
Safety and immunogenicity of an inactivated recombinant Newcastle disease virus vaccine expressing SARS-CoV-2 spike: Results of a randomized vaccine-controlled phase I ADAPTCOV trial in Brazil.Vaccine. 2025 Apr 11;52:126680. doi: 10.1016/j.vaccine.2024.126680. Epub 2025 Mar 3. Vaccine. 2025. PMID: 40037239 Clinical Trial.
Cited by
-
Moloney Murine Leukemia Virus-like Nanoparticles Pseudo-Typed with SARS-CoV-2 RBD for Vaccination Against COVID-19.Int J Mol Sci. 2025 Jul 4;26(13):6462. doi: 10.3390/ijms26136462. Int J Mol Sci. 2025. PMID: 40650237 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

