MARCH8 Restricts RSV Replication by Promoting Cellular Apoptosis Through Ubiquitin-Mediated Proteolysis of Viral SH Protein
- PMID: 39772241
- PMCID: PMC11680241
- DOI: 10.3390/v16121935
MARCH8 Restricts RSV Replication by Promoting Cellular Apoptosis Through Ubiquitin-Mediated Proteolysis of Viral SH Protein
Abstract
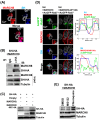
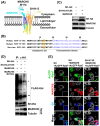
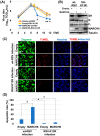
Numerous host factors function as intrinsic antiviral effectors to attenuate viral replication. MARCH8 is an E3 ubiquitin ligase that has been identified as a host restriction factor that inhibits the replication of various viruses. This study elucidated the mechanism by which MARCH8 restricts respiratory syncytial virus (RSV) replication through selective degradation of the viral small hydrophobic (SH) protein. We demonstrated that MARCH8 directly interacts with RSV-SH and catalyzes its ubiquitination at lysine 13, leading to SH degradation via the ubiquitin-lysosomal pathway. Functionally, MARCH8 expression enhances RSV-induced apoptosis through SH degradation, ultimately reducing viral titers. Conversely, an RSV strain harboring the SH-K13R mutation exhibited prolonged SH protein stability and attenuated apoptosis in infected cells, even in the presence of MARCH8. Targeted depletion of MARCH8 enhances cellular survival and potentially increases viral persistence. These findings demonstrate that MARCH8 promotes the early elimination of virus-infected cells by abrogating the anti-apoptotic function of SH, thereby reducing viral transmission. Our study provides novel insights into the interplay between host restriction factors and viral evasion strategies, potentially providing new therapeutic approaches for RSV infections.
Keywords: MARCH8; apoptosis; respiratory syncytial virus; small hydrophobic protein; ubiquitination.
Conflict of interest statement
T.K. is currently an employee of Kanto Chemical Co., Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures


Similar articles
-
GBP5 Is an Interferon-Induced Inhibitor of Respiratory Syncytial Virus.J Virol. 2020 Oct 14;94(21):e01407-20. doi: 10.1128/JVI.01407-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32796072 Free PMC article.
-
MARCH8 inhibits influenza A virus infection by targeting viral M2 protein for ubiquitination-dependent degradation in lysosomes.Nat Commun. 2021 Jul 20;12(1):4427. doi: 10.1038/s41467-021-24724-2. Nat Commun. 2021. PMID: 34285233 Free PMC article.
-
Identification of Respiratory Syncytial Virus Nonstructural Protein 2 Residues Essential for Exploitation of the Host Ubiquitin System and Inhibition of Innate Immune Responses.J Virol. 2016 Jun 24;90(14):6453-6463. doi: 10.1128/JVI.00423-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147743 Free PMC article.
-
New insights on the viral and host factors contributing to the airway pathogenesis caused by the respiratory syncytial virus.Crit Rev Microbiol. 2016 Sep;42(5):800-12. doi: 10.3109/1040841X.2015.1055711. Epub 2015 Jun 29. Crit Rev Microbiol. 2016. PMID: 26119025 Review.
-
Involvement of E3 Ubiquitin Ligases in Viral Infections of the Human Host.Viral Immunol. 2024 Nov;37(9):419-431. doi: 10.1089/vim.2024.0068. Epub 2024 Oct 29. Viral Immunol. 2024. PMID: 39469796 Review.
Cited by
-
Insights into the Currently Available Drugs and Investigational Compounds Against RSV with a Focus on Their Drug-Resistance Profiles.Viruses. 2025 May 30;17(6):793. doi: 10.3390/v17060793. Viruses. 2025. PMID: 40573384 Free PMC article. Review.
References
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., A Madhi S., Omer S.B., Simões E.A.F., Campbell H., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

