A Subunit Vaccine Harboring the Fusion Capsid Proteins of Porcine Circovirus Types 2, 3, and 4 Induces Protective Immune Responses in a Mouse Model
- PMID: 39772270
- PMCID: PMC11728783
- DOI: 10.3390/v16121964
A Subunit Vaccine Harboring the Fusion Capsid Proteins of Porcine Circovirus Types 2, 3, and 4 Induces Protective Immune Responses in a Mouse Model
Abstract
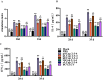
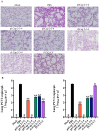
Coinfections with porcine circovirus types 2, 3, and 4 (PCV2, PCV3, and PCV4) are increasingly being detected in the swine industry. However, there is no commercially available vaccine which prevents coinfection with PCV2, PCV3, and PCV4. The development of a vaccine expressing capsid (Cap) fusion proteins of multiple PCVs represents a promising approach for broadly preventing infection with PCVs. In this study, we developed a PCV subunit vaccine candidate (Cap 2-3-4) by predicting, screening, and fusing antigenic epitopes of Cap proteins of PCV2, PCV3, and PCV4. Immunoprotection assays showed that the prokaryotic expression of Cap 2-3-4 could effectively induce high levels of PCV2, PCV3, and PCV4 Cap-specific antibodies and successfully neutralize both PCV2 and PCV3. Furthermore, Cap 2-3-4 demonstrated a potent ability to activate cellular immunity and thus prevent lung damage in mice. This study provides a new option for the development of broad vaccines against PCVs.
Keywords: antigenic epitope; capsid; fusion protein; immunogenicity; porcine circovirus; subunit vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Chen N., Huang Y., Ye M., Li S., Xiao Y., Cui B., Zhu J. Co-infection status of classical swine fever virus (CSFV), porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circoviruses (PCV2 and PCV3) in eight regions of China from 2016 to 2018. Infect. Genet. Evol. 2019;68:127–135. doi: 10.1016/j.meegid.2018.12.011. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 32302875, 82273837/National Natural Science Foundation of China
- 202303021221091/Fundamental Research Program of Shanxi Province
- 202102140601020/Shanxi Provincial Key Research and Development Program
- 2021L130/Science and Technology Innovation Program for Higher Education Institutions in Shanxi Province
- 2021BQ79/Start-up Fund for doctoral research, Shanxi Agricultural University
LinkOut - more resources
Full Text Sources
Miscellaneous

