Avian Reovirus: From Molecular Biology to Pathogenesis and Control
- PMID: 39772272
- PMCID: PMC11728826
- DOI: 10.3390/v16121966
Avian Reovirus: From Molecular Biology to Pathogenesis and Control
Abstract
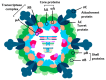
Avian reoviruses (ARVs) represent a significant economic burden on the poultry industry due to their widespread prevalence and potential pathogenicity. These viruses, capable of infecting a diverse range of avian species, can lead to a variety of clinical manifestations, most notably tenosynovitis/arthritis. While many ARV strains are asymptomatic, pathogenic variants can cause severe inflammation and tissue damage in organs such as the tendons, heart, and liver. In broilers and turkeys, ARVs can induce severe arthritis/tenosynovitis, characterized by swollen hock joints and lesions in the gastrocnemius tendons. Additionally, ARVs have been implicated in other diseases, although their precise role in these conditions remains to be fully elucidated. In recent years, ARV cases have surged in the United States, emphasizing the need for effective control measures. Routine vaccination with commercial or autogenous vaccines is currently the primary strategy for mitigating ARV's impact. Future research efforts should focus on enhancing our understanding of ARV-induced pathogenesis, identifying host factors that influence disease severity, and developing novel vaccines based on ongoing surveillance of circulating ARV strains. This review aims to explore the molecular aspects of ARV, including virus structure, replication, molecular epidemiology, the roles of its encoded proteins in host pathogenesis, and the immune response to ARV infection. Furthermore, we discuss the diagnostic approaches of avian reovirus and the potential biosecurity measures and vaccination trials in combating ARV and developing effective antiviral strategies.
Keywords: avian reovirus; epidemiology; immune response; pathogenesis; replication; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











References
-
- Giangaspero M., Vanopdenbosch E., Nishikawa H., Tabbaa D. Prevalence of antibodies against respiratory viruses (parainfluenza virus type 3, respiratory syncytial virus, reovirus and adenovirus) in relation to productivity in Syrian Awassi sheep. Trop. Anim. Health Prod. 1997;29:83–91. doi: 10.1007/BF02632323. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

