Demographic-Based Personalized Left Ventricular Hypertrophy Thresholds for Hypertrophic Cardiomyopathy Diagnosis
- PMID: 39772357
- PMCID: PMC12461611
- DOI: 10.1016/j.jacc.2024.10.082
Demographic-Based Personalized Left Ventricular Hypertrophy Thresholds for Hypertrophic Cardiomyopathy Diagnosis
Abstract
Background: Hypertrophic cardiomyopathy (HCM) is a leading cause of sudden cardiac death. Current diagnosis emphasizes the detection of left ventricular hypertrophy (LVH) using a fixed threshold of ≥15-mm maximum wall thickness (MWT). This study proposes a method that considers individual demographics to adjust LVH thresholds as an alternative to a 1-size-fits-all approach.
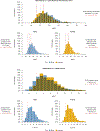
Methods: Left ventricular MWT was measured in 3 cohorts: a Reference Cohort of healthy adults (n = 5,067, no comorbidities), a Population Cohort (n = 43,239, with comorbidities), and an HCM Cohort from 6 international centers (n = 2,424). Measurement used cardiovascular magnetic resonance (CMR) and a validated artificial intelligence algorithm. The Reference Cohort was used to developed demographically adjusted LVH thresholds, and individualized z-scores based on age, sex, and body surface area (BSA), which were used to explore the other cohorts.
Results: The traditional ≥15-mm threshold classified 4.3% (n = 1,854) of the Population Cohort as hypertrophic, with a significant sex skew (89% male). Demographic-adjusted LVH thresholds (range: 10-17 mm) reduced ascertainment to 2.2% (n = 945), reducing the sex skew (56% male). Similar reductions in bias with height, weight, and age also occurred. The HCM cohort was found to have a 2:1 male-to-female ratio. A significant proportion of patients received diagnoses of HCM despite having MWT below the traditional LVH threshold (<15 mm): 27% of female individuals and 18% of male individuals. Using demographic-adjusted LVH thresholds reduced these proportions to 7% of female individuals and 15% of male individuals (P < 0.0001). Female patients had lower absolute MWT (18 mm vs 19 mm; P < 0.001) but higher MWT z-scores (5.1 vs 4.5; P = 0.05).
Conclusions: Age, sex, and body size influence the normal heart MWT. Using a fixed LVH threshold ≥15 mm biases LVH ascertainment in both population and HCM cohorts. A demographic-adjusted approach for LVH improves ascertainment and diagnostic accuracy.
Keywords: cardiac magnetic resonance; hypertrophic cardiomyopathy; left ventricular hypertrophy.
Copyright © 2025 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures Part of the work reported in this publication was funded by the Italian Ministry of Health (RC-2024-2789983; PNRR-MAD-2022-12375700; PNRR The RESPECT Project; PNRR PE0000019; HEAL ITALIA). Part of the work reported in this publication was funded by the European Union (Next Generation EU - NRRP M6C2). The funders played no role in the study design, in the collection, analysis or interpretation of data, in the report’s writing, or in the decision to submit the article for publication. Dr Shiwai was supported by a British Heart Foundation Hope for Hearts grant and is a paid consultant for MyCardium AI Ltd. Dr Topriceanu was supported by a British Cardiovascular Society-Heart Research UK fellowship. Dr Davies is funded by the British Heart Foundation (BHF) Accelerator Award (AA/18/6/34223). Dr Ditaranto is supported by a grant from Pfizer (52524547); and is supported by the Italian Ministry of Health. Dr Owens has received consultancy fees from Bristol Myers Squibb, Cytokinetics, Pfizer, Lexicon, Lexeo, Stealth, Edgewise, Tenaya, BioMarin, and CorVista. Dr Raman has received funding support from the British Heart Foundation (BHF) Oxford Centre of Research Excellence (CRE) and National Institute for Health and Care Research (NIHR) Oxford Biomedical Research Centre; and has received payment or honoraria for lectures, presentations, or educational events from Axcella Therapeutics. Dr Kramer is supported by grants from Cytokinetics and Bristol Myers Squibb; and has received consulting fees from Biomarin and Bristol Myers Squibb. Dr Lopes is supported by a Medical Research Council UK Research and Innovation Clinical Academic Research Partnership award (MR/T005181/1). Dr Captur is supported by the British Heart Foundation special project grant (SP/20/2/34841), the National Institute for Health and Care Research innovation for innovation iFAST grant, and the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre. Dr Moon has received funding directly and indirectly from the National Institutes of Health and Care Research Biomedical Research Centres at University College London Hospitals and Barts Health NHS trusts; is the chief executive officer of MyCardium AI Ltd; and has served on advisory boards for Sanofi and Genzyme. The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

References
-
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. - PubMed
-
- Arbelo E, Protonotarios A, Gimeno JR, et al. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44(37):3503–3626. - PubMed
-
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76:e159–e240. - PubMed
-
- Henry WL, Clark CE, Epstein SE. Asymmetric septal hypertrophy: echocardiographic identification of the pathognomonic anatomic abnormality of IHSS. Circulation. 1973;47:225–233. - PubMed
-
- Kansal S, Roitman D, Sheffield LT. Interventricular septal thickness and left ventricular hypertrophy: an echocardiographic study. Circulation. 1979;60:1058–1065. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

