Combining Photochemical Oxyfunctionalization and Enzymatic Catalysis for the Synthesis of Chiral Pyrrolidines and Azepanes
- PMID: 39772597
- PMCID: PMC11744798
- DOI: 10.1021/acs.joc.4c02228
Combining Photochemical Oxyfunctionalization and Enzymatic Catalysis for the Synthesis of Chiral Pyrrolidines and Azepanes
Abstract
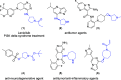
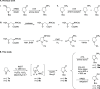
Chiral heterocyclic alcohols and amines are frequently used building blocks in the synthesis of fine chemicals and pharmaceuticals. Herein, we report a one-pot photoenzymatic synthesis route for N-Boc-3-amino/hydroxy-pyrrolidine and N-Boc-4-amino/hydroxy-azepane with up to 90% conversions and >99% enantiomeric excess. The transformation combines a photochemical oxyfunctionalization favored for distal C-H positions with a stereoselective enzymatic transamination or carbonyl reduction step. Our study demonstrates a mild and operationally simple asymmetric synthesis workflow from easily available starting materials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals: Miniperspective. J. Med. Chem. 2014, 57 (24), 10257–10274. 10.1021/jm501100b. - DOI - PubMed
- Kittakoop P.; Mahidol C.; Ruchirawat S. Alkaloids as Important Scaffolds in Therapeutic Drugs for the Treatments of Cancer, Tuberculosis, and Smoking Cessation. Curr. Top. Med. Chem. 2013, 14 (2), 239–252. 10.2174/1568026613666131216105049. - DOI - PubMed
- Roughley S. D.; Jordan A. M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54 (10), 3451–3479. 10.1021/jm200187y. - DOI - PubMed
-
- Zhang J.; Wei Y.; Zhou X.; Yang K.; Sun Y.. PARP1 inhibitor and application thereof. CN 117447449A, 2022.
- Freeman D. B.; Hopkins T. D.; Mikochik P. J.; Vacca J. P.; Gao H.; Naylor-Olsen A.; Rudra S.; Li H.; Pop M. S.; Villagomez R. A.; Lee C.; Li H.; Zhou M.; Saffran D. C.; Rioux N.; Hood T. R.; Day M. A. L.; McKeown M. R.; Lin C. Y.; Bischofberger N.; Trotter B. W. Discovery of KB-0742, a Potent, Selective, Orally Bioavailable Small Molecule Inhibitor of CDK9 for MYC-Dependent Cancers. J. Med. Chem. 2023, 66 (23), 15629–15647. 10.1021/acs.jmedchem.3c01233. - DOI - PMC - PubMed
- Petri G. L.; Raimondi M. V.; Spanò V.; Holl R.; Barraja P.; Montalbano A. Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Top. Curr. Chem. 2021, 379 (5), 3410.1007/s41061-021-00347-5. - DOI - PMC - PubMed
- Mikochik P.; Vacca J.; Freeman D.; Tasker A.. Compounds, Compositions, and Methods for Modulating Cdk9 Activity. CA 3118472 A1, 2020.
- McGrath N. A.; Brichacek M.; Njardarson J. T. A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Educ. 2010, 87 (12), 1348–1349. 10.1021/ed1003806. - DOI
-
- Hughes D. L. Patent Review of Manufacturing Routes to Fifth-Generation Cephalosporin Drugs. Part 2, Ceftaroline Fosamil and Ceftobiprole Medocaril. Org. Process Res. Dev. 2017, 21 (6), 800–815. 10.1021/acs.oprd.7b00143. - DOI
-
- Hoegenauer K.; Soldermann N.; Zécri F.; Strang R. S.; Graveleau N.; Wolf R. M.; Cooke N. G.; Smith A. B.; Hollingworth G. J.; Blanz J.; Gutmann S.; Rummel G.; Littlewood-Evans A.; Burkhart C. Discovery of CDZ173 (Leniolisib), Representing a Structurally Novel Class of PI3K Delta-Selective Inhibitors. ACS Med. Chem. Lett. 2017, 8 (9), 975–980. 10.1021/acsmedchemlett.7b00293. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

