Structural insights into hybridoma-derived neutralizing monoclonal antibodies against Omicron BA.5 and XBB.1.16 variants of SARS-CoV-2
- PMID: 39772622
- PMCID: PMC11852929
- DOI: 10.1128/jvi.01307-24
Structural insights into hybridoma-derived neutralizing monoclonal antibodies against Omicron BA.5 and XBB.1.16 variants of SARS-CoV-2
Abstract
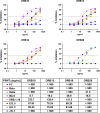
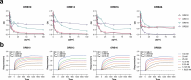
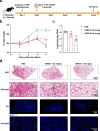
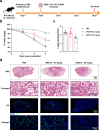
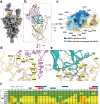
The emergence of novel variants of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) continues to pose an ongoing challenge for global public health services, highlighting the urgent need for effective therapeutic interventions. Neutralizing monoclonal antibodies (mAbs) are a major therapeutic strategy for the treatment of COVID-19 and other viral diseases. In this study, we employed hybridoma technology to generate mAbs that target the BA.5 receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. Through a comprehensive screening process, we identified four mAbs capable of effectively neutralizing BA.5, XBB.1.16, and related variant infections in vitro, among which ORB10 was found to neutralize BA.5 variants with a plaque reduction neutralization test (PRNT50) of 8.7 ng/mL. Additionally, competitive binding assays, sequencing of heavy and light chain variable regions, and binding kinetics characterization provided insights into the epitopes and binding affinities of the identified mAbs. Moreover, in vivo experiments in the K18-hACE2 mouse model demonstrated the protective efficacy of ORB10 against both BA.5 and XBB.1.16 variants. Finally, cryo-electron microscopy structural analysis of the ORB10-RBD complex identified key residues involved in the antibody-antigen interactions, providing insights into the molecular mechanisms of neutralization and immune escape of SARS-CoV-2 Omicron variants from mAbs.
Importance: The ongoing evolution of SARS-CoV-2 has led to the emergence of variants capable of evading immune responses elicited by natural infection and vaccination, especially the highly transmissible and immune-evasive Omicron variants. This study generated and characterized a panel of monoclonal antibodies (mAbs) specifically targeting the RBD of the Omicron BA.5 variant, of which the ORB10 showed efficacy against Omicron BA.5 and XBB.1.16 variants both in vitro and in vivo. Cryo-EM structural analysis further elucidated the binding epitope interactions and neutralization mechanism between ORB10 and the BA.5 RBD protein. This study enhances our understanding of antibody-mediated neutralization of SARS-CoV-2 and provides valuable insights into the development of effective therapeutic strategies to combat ongoing SARS-CoV-2 variant infections.
Keywords: K18-hACE2 mouse model; Omicron variants; SARS-CoV-2; cryo-EM structure; neutralizing antibody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

