Functions of nitroreductases in mycobacterial physiology and drug susceptibility
- PMID: 39772630
- PMCID: PMC11841060
- DOI: 10.1128/jb.00326-24
Functions of nitroreductases in mycobacterial physiology and drug susceptibility
Abstract
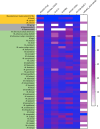
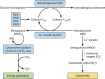
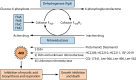
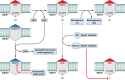
Tuberculosis is a respiratory infection that is caused by members of the Mycobacterium tuberculosis complex, with M. tuberculosis (Mtb) being the predominant cause of the disease in humans. The approval of pretomanid and delamanid, two nitroimidazole-based compounds, for the treatment of tuberculosis encourages the development of more nitro-containing drugs that target Mtb. Similar to the nitroimidazoles, many antimycobacterial nitro-containing scaffolds are prodrugs that require reductive activation into metabolites that inhibit the growth of the pathogen. This reductive activation is mediated by mycobacterial nitroreductases, leading to the hypothesis that these nitroreductases contribute to the specificity of the nitro prodrugs for mycobacteria. In addition to their prodrug-activating activities, these nitroreductases have different native activities that support the growth of the bacteria. This review summarizes the activities of different mycobacterial nitroreductases with respect to their activation of different nitro prodrugs and highlights their physiological functions in the bacteria.
Keywords: Mycobacterium; antimicrobials; nitroreductase.
Conflict of interest statement
R.B.A. is the founder and owner of Tarn Biosciences, Inc., a company that is working to develop new antimycobacterial drugs.
Figures
References
-
- Cellitti SE, Shaffer J, Jones DH, Mukherjee T, Gurumurthy M, Bursulaya B, Boshoff HI, Choi I, Nayyar A, Lee YS, Cherian J, Niyomrattanakit P, Dick T, Manjunatha UH, Barry CE 3rd, Spraggon G, Geierstanger BH. 2012. Structure of Ddn, the deazaflavin-dependent nitroreductase from Mycobacterium tuberculosis involved in bioreductive activation of PA-824. Structure 20:101–112. doi: 10.1016/j.str.2011.11.001 - DOI - PMC - PubMed
-
- Gurumurthy M, Mukherjee T, Dowd CS, Singh R, Niyomrattanakit P, Tay JA, Nayyar A, Lee YS, Cherian J, Boshoff HI, Dick T, Barry CE III, Manjunatha UH. 2012. Substrate specificity of the deazaflavin-dependent nitroreductase from Mycobacterium tuberculosis responsible for the bioreductive activation of bicyclic nitroimidazoles. FEBS J 279:113–125. doi: 10.1111/j.1742-4658.2011.08404.x - DOI - PMC - PubMed
-
- Rifat D, Li SY, Ioerger T, Shah K, Lanoix JP, Lee J, Bashiri G, Sacchettini J, Nuermberger E. 2020. Mutations in fbiD (Rv2983) as a novel determinant of resistance to pretomanid and delamanid in Mycobacterium tuberculosis. Antimicrob Agents Chemother 65:e01948-20. doi: 10.1128/AAC.01948-20 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

