Stability and Properties of Ultraviolet Filter Avobenzone under Its Diketo/Enol Tautomerization Induced by Molecular Encapsulation with β-Cyclodextrin
- PMID: 39772649
- PMCID: PMC11755785
- DOI: 10.1021/acs.langmuir.4c04108
Stability and Properties of Ultraviolet Filter Avobenzone under Its Diketo/Enol Tautomerization Induced by Molecular Encapsulation with β-Cyclodextrin
Abstract
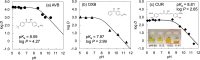
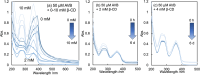
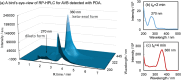
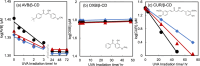
Inclusion complexation of the sunscreen ingredient avobenzone (AVB) with β-cyclodextrin (β-CD) was investigated to improve its aqueous solubility and photostability; another ultraviolet (UV) filter, oxybenzone (OXB), and the phytochemical antioxidant curcumin (CUR) served as a comparison. In this study, the 1-octanol/water partition coefficients, acid dissociation constants, phase-solubility diagrams with β-CD, and ultraviolet-visible (UV-vis) spectral changes induced by UVA1 (365 nm) irradiation were evaluated. β-CD at concentrations 50-100 times that of AVB most effectively protected the photostability of AVB. Additionally, an UVA1-insensitive species with a diketo tautomer, which has an UVC-absorbing band and the potential to cause photodegradation, was stored in the inclusion complex. Acetonitrile-water mixtures at various volume ratios were screened to mimic the internal cavity of β-CD for the AVB tautomeric species using nuclear magnetic resonance (NMR) spectral integrals for the components. The results indicated that β-CD provides a hydrophobic environment similar to that of a 40-50% acetonitrile aqueous solution and enhances the photostability of AVB. However, excess β-CD induced a hyperchromic effect on the diketo tautomer. Aggregation of the AVB/β-CD inclusion complexes at β-CD concentrations of ≥2 mM enhances UVC band absorption. To avoid excess β-CD, a molar ratio of 50-100 of β-CD to AVB is recommended as the optimal composition. This study newly exhibited that the cavity of β-CD mitigates the reactivity of UVA1 toward AVB by inducing the diketo tautomer form of AVB within the cavity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- United States Food and Drug Administration (U.S. FDA) . OTC Monographs@FDA; U.S. FDA: Silver Spring, MD, 2021; https://www.accessdata.fda.gov/drugsatfda_docs/omuf/OTCMonograph_M020-Su... (accessed Aug 9, 2024).
-
- United States Food and Drug Administration (U.S. FDA) . Questions and Answers: FDA Posts Deemed Final Order and Proposed Order for Over-the-Counter Sunscreen; U.S. FDA: Silver Spring, MD, 2022; https://www.fda.gov/drugs/understanding-over-counter-medicines/questions... (accessed Aug 9, 2024).
-
- Dicken J. E.Over-the-counter drugs. Information of FDA’s regulation of most OTC drugs. Report to the Congressional Committees; U.S. Government Accountability Office (GAO): Washington, D.C., 2020; GAO-20-572, https://www.gao.gov/assets/gao-20-572.pdf (accessed Aug 9, 2024).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

