Delay of innate immune responses following influenza B virus infection affects the development of a robust antibody response in ferrets
- PMID: 39772665
- PMCID: PMC11796412
- DOI: 10.1128/mbio.02361-24
Delay of innate immune responses following influenza B virus infection affects the development of a robust antibody response in ferrets
Abstract
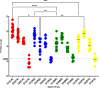
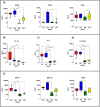
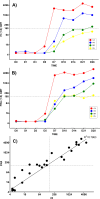
Due to its natural influenza susceptibility, clinical signs, transmission, and similar sialic acid residue distribution, the ferret is the primary animal model for human influenza research. Antibodies generated following infection of ferrets with human influenza viruses are used in surveillance to detect antigenic drift and cross-reactivity with vaccine viruses and circulating strains. Inoculation of ferrets, with over 1,500 human clinical influenza isolates (1998-2019) resulted in lower antibody responses (HI <1:160) to 86% (387 out of 448) influenza B viruses (IBVs) compared to 2.7% (30 out of 1,094) influenza A viruses (IAVs). Here, we show that the immune responses in ferrets inoculated with IBV were delayed and reduced compared to IAV. Innate gene expression in the upper respiratory tract and blood indicated that IAV generated a strong inflammatory response, including an early activation of the interferon (IFN), whereas IBV elicited a delayed and reduced response. Serum levels of cytokines and IFNs were all much higher following IAV infection than IBV infection. Pro-inflammatory, IFN, TH1/TH2, and T-effector proteins were significantly higher in sera of IAV-infected than IBV-infected ferrets over 28 days following the challenge. Serum levels of Type-I/II/III IFNs were detected following IAV infection throughout this period, whereas Type-III IFN was only late for IBV. An early increase in IFN-lambda corresponded to gene expression following IAV infection. Reduced innate immune responses following IBV infection reflected the subsequent delayed and reduced serum antibodies. These findings may help in understanding the antibody responses in humans following influenza vaccination or infection and consideration of potential addition of innate immunomodulators to overcome low responses.
Importance: The ferret is the primary animal model for human influenza research. Using a ferret model, we studied the differences in both innate and adaptive immune responses following infection with influenza A and B viruses (IAV and IBV). Antibodies generated following infection of ferrets is used for surveillance assays to detect antigenic drift and cross-reactivity with vaccine viruses and circulating influenza strains. IAV infection of ferrets to generate these reagents resulted in a strong antibody response, but IBV infection generated weak antibody responses. In this study using influenza-infected ferrets, we found that IAV resulted in an early activation of the interferon (IFN) and pro-inflammatory response, whereas IBV showed a delay and reduction in these responses. Serum levels of IFNs and other cytokines or chemokines were much higher in ferrets following IAV infection. These reduced innate responses were reflected the subsequent delayed and reduced antibody responses to IBV in the sera. These findings may help in understanding low antibody responses in humans following influenza B vaccination and infection and may warrant the use of innate immunomodulators to overcome these weak responses.
Keywords: adaptive; ferret; focus reduction assay; hemagglutinin inhibition; influenza B; innate; interferon; multiplex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ng PSK, Böhm R, Hartley-Tassell LE, Steen JA, Wang H, Lukowski SW, Hawthorne PL, Trezise AEO, Coloe PJ, Grimmond SM, Haselhorst T, von Itzstein M, Paton AW, Paton JC, Jennings MP. 2014. Ferrets exclusively synthesize Neu5Ac and express naturally humanized influenza A virus receptors. Nat Commun 5:5750. doi: 10.1038/ncomms6750 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

