Plasticity of human resilience mechanisms
- PMID: 39772669
- PMCID: PMC11708882
- DOI: 10.1126/sciadv.adq8336
Plasticity of human resilience mechanisms
Abstract
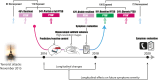
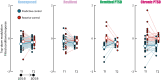
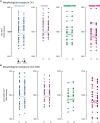
The hippocampus's vulnerability to trauma-induced stress can lead to pathophysiological disturbances that precipitate the development of posttraumatic stress disorder (PTSD). The mechanisms of resilience that foster remission and mitigate the adverse effects of stress remain unknown. We analyzed the evolution of hippocampal morphology between 2016/2017 and 2018/2019, as well as the memory control mechanisms crucial for trauma resilience. Participants were individuals exposed to the 2015 Paris terrorist attacks (N = 100), including chronic (N = 34) and remitted (N = 19) PTSD, and nonexposed (N = 72). We found that normalization of inhibitory control processes, which regulate the resurgence of intrusive memories in the hippocampus, not only predicted PTSD remission but also preceded a reduction in traumatic memories. Improvement in control mechanisms was associated with the interruption of stress-induced atrophy in a hippocampal region that includes the dentate gyrus. Human resilience to trauma is characterized by the plasticity of memory control circuits, which interacts with hippocampal neuroplasticity.
Figures



References
-
- Kalisch R., Baker D. G., Basten U., Boks M. P., Bonanno G. A., Brummelman E., Chmitorz A., Fernàndez G., Fiebach C. J., Galatzer-Levy I., Geuze E., Groppa S., Helmreich I., Hendler T., Hermans E. J., Jovanovic T., Kubiak T., Lieb K., Lutz B., Müller M. B., Murray R. J., Nievergelt C. M., Reif A., Roelofs K., Rutten B. P. F., Sander D., Schick A., Tüscher O., Diest I., van Harmelen A.-L., Veer I. M., Vermetten E., Vinkers C. H., Wager T. D., Walter H., Wessa M., Wibral M., Kleim B., The resilience framework as a strategy to combat stress-related disorders. Nat. Hum. Behav. 1, 784–790 (2017). - PubMed
-
- van Rooij S. J. H., Santos J. L., Hinojosa C. A., Ely T. D., Harnett N. G., Murty V. P., Lebois L. A. M., Jovanovic T., House S. L., Bruce S. E., Beaudoin F. L., An X., Neylan T. C., Clifford G. D., Linnstaedt S. D., Germine L. T., Bollen K. A., Rauch S. L., Haran J. P., Storrow A. B., Lewandowski C., Musey P. I., Hendry P. L., Sheikh S., Jones C. W., Punches B. E., Swor R. A., Pascual J. L., Seamon M. J., Harris E., Pearson C., Peak D. A., Merchant R. C., Domeier R. M., Rathlev N. K., O’Neil B. J., Sanchez L. D., Joormann J., Pizzagalli D. A., Sheridan J. F., Harte S. E., Kessler R. C., Koenen K. C., McLean S. A., Ressler K. J., Stevens J. S., Defining the r factor for post-trauma resilience and its neural predictors. Nat. Ment. Health 2, 680–693 (2024).
-
- Yehuda R., LeDoux J., Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron 56, 19–32 (2007). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

