Differential encoding of mammalian proprioception by voltage-gated sodium channels
- PMID: 39772670
- PMCID: PMC11708877
- DOI: 10.1126/sciadv.ads6660
Differential encoding of mammalian proprioception by voltage-gated sodium channels
Abstract
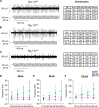
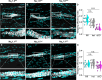
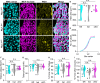
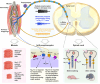
Animals requiring purposeful movement for survival are endowed with mechanoreceptors, called proprioceptors, that provide essential sensory feedback from muscles and joints to spinal cord circuits, which modulates motor output. Despite the essential nature of proprioceptive signaling in daily life, the mechanisms governing proprioceptor activity are poorly understood. Here, we identified nonredundant roles for two voltage-gated sodium channels (NaVs), NaV1.1 and NaV1.6, in mammalian proprioception. Deletion of NaV1.6 in somatosensory neurons (NaV1.6cKO mice) causes severe motor deficits accompanied by loss of proprioceptive transmission, which contrasts with our previous findings using similar mouse models to target NaV1.1 (NaV1.1cKO). In NaV1.6cKO animals, we observed impairments in proprioceptor end-organ structure and a marked reduction in skeletal muscle myofiber size that were absent in NaV1.1cKO mice. We attribute the differential contributions of NaV1.1 and NaV1.6 to distinct cellular localization patterns. Collectively, we provide evidence that NaVs uniquely shape neural signaling within a somatosensory modality.
Figures






Update of
-
Differential encoding of mammalian proprioception by voltage-gated sodium channels.bioRxiv [Preprint]. 2024 Aug 28:2024.08.27.609982. doi: 10.1101/2024.08.27.609982. bioRxiv. 2024. Update in: Sci Adv. 2025 Jan 10;11(2):eads6660. doi: 10.1126/sciadv.ads6660. PMID: 39253497 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

