A spatial code for temporal information is necessary for efficient sensory learning
- PMID: 39772691
- PMCID: PMC11708902
- DOI: 10.1126/sciadv.adr6214
A spatial code for temporal information is necessary for efficient sensory learning
Abstract
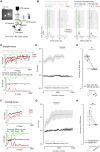
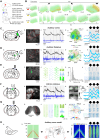
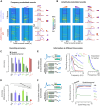
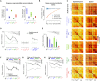
The temporal structure of sensory inputs contains essential information for their interpretation. Sensory cortex represents these temporal cues through two codes: the temporal sequences of neuronal activity and the spatial patterns of neuronal firing rate. However, it is unknown which of these coexisting codes causally drives sensory decisions. To separate their contributions, we generated in the mouse auditory cortex optogenetically driven activity patterns differing exclusively along their temporal or spatial dimensions. Mice could rapidly learn to behaviorally discriminate spatial but not temporal patterns. Moreover, large-scale neuronal recordings across the auditory system revealed that the auditory cortex is the first region in which spatial patterns efficiently represent temporal cues on the timescale of several hundred milliseconds. This feature is shared by the deep layers of neural networks categorizing time-varying sounds. Therefore, the emergence of a spatial code for temporal sensory cues is a necessary condition to efficiently associate temporally structured stimuli with decisions.
Figures



References
-
- Saberi K., Perrott D. R., Cognitive restoration of reversed speech. Nature 398, 760 (1999). - PubMed
-
- Rosen S., Temporal information in speech: Acoustic, auditory and linguistic aspects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 336, 367–373 (1992). - PubMed
-
- Jadhav S. P., Wolfe J., Feldman D. E., Sparse temporal coding of elementary tactile features during active whisker sensation. Nat. Neurosci. 12, 792–800 (2009). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

