A positive feedback loop between germ cells and gonads induces and maintains sexual reproduction in a cnidarian
- PMID: 39772697
- PMCID: PMC11708894
- DOI: 10.1126/sciadv.adq8220
A positive feedback loop between germ cells and gonads induces and maintains sexual reproduction in a cnidarian
Abstract
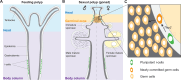
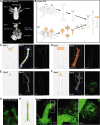
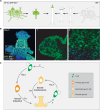
The fertile gonad includes cells of two distinct developmental origins: the somatic mesoderm and the germ line. How somatic and germ cells interact to develop and maintain fertility is not well understood. Here, using grafting experiments and transgenic reporter animals, we find that a specific part of the gonad-the germinal zone-acts as a sexual organizer to induce and maintain de novo germ cells and somatic gonads in the cnidarian Hydractinia symbiolongicarpus. Germ cells express a member of the transforming growth factor-β family, Gonadless (Gls), that induces gonad morphogenesis. Loss of Gls resulted in animals lacking gonads but having nonproliferative germ cells. We propose that primary germ cells drive gonad development though Gls secretion. The germinal zone in the newly formed gonad provides positive feedback to induce secondary germ cells by activating Tfap2 in resident pluripotent stem cells. The contribution of germ cell signaling to the patterning of somatic gonadal tissue may be a general animal feature.
Figures


Similar articles
-
Transcription factor AP2 controls cnidarian germ cell induction.Science. 2020 Feb 14;367(6479):757-762. doi: 10.1126/science.aay6782. Science. 2020. PMID: 32054756 Free PMC article.
-
Transcriptional Regulation of Müllerian Inhibiting Substance (MIS) and Establishment of a Gonadal Somatic Cell Line Using mis-GFP Transgenic Medaka (Oryzias latipes).Front Endocrinol (Lausanne). 2020 Sep 29;11:578885. doi: 10.3389/fendo.2020.578885. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33133021 Free PMC article.
-
Distinct activities of the germline and somatic reproductive tissues in the regulation of Caenorhabditis elegans' longevity.Genetics. 2008 Jan;178(1):513-26. doi: 10.1534/genetics.107.083253. Genetics. 2008. PMID: 18202391 Free PMC article.
-
Somatic gonadal cells: the supporting cast for the germline.Genesis. 2011 Oct;49(10):753-75. doi: 10.1002/dvg.20784. Genesis. 2011. PMID: 21735540 Review.
-
The Central Role of Cadherins in Gonad Development, Reproduction, and Fertility.Int J Mol Sci. 2020 Nov 4;21(21):8264. doi: 10.3390/ijms21218264. Int J Mol Sci. 2020. PMID: 33158211 Free PMC article. Review.
Cited by
-
A whole-body atlas of BMP signaling activity in an adult sea anemone.BMC Biol. 2025 Feb 21;23(1):49. doi: 10.1186/s12915-025-02150-w. BMC Biol. 2025. PMID: 39984987 Free PMC article.
References
-
- Extavour C. G., Akam M., Mechanisms of germ cell specification across the metazoans: Epigenesis and preformation. Development 130, 5869–5884 (2003). - PubMed
-
- Lochab A. K., Extavour C. G., Bone Morphogenetic Protein (BMP) signaling in animal reproductive system development and function. Dev. Biol. 427, 258–269 (2017). - PubMed
-
- Jemc J. C., Somatic gonadal cells: The supporting cast for the germline. Genesis 49, 753–775 (2011). - PubMed
-
- Rios-Rojas C., Bowles J., Koopman P., On the role of germ cells in mammalian gonad development: Quiet passengers or back-seat drivers? Reproduction 149, R181–R191 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

