Small RNA CjNC110 regulates the activated methyl cycle to enable optimal chicken colonization by Campylobacter jejuni
- PMID: 39772717
- PMCID: PMC11774046
- DOI: 10.1128/msphere.00832-24
Small RNA CjNC110 regulates the activated methyl cycle to enable optimal chicken colonization by Campylobacter jejuni
Abstract
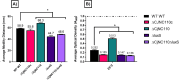
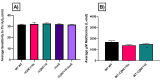
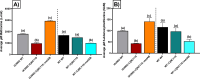
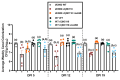
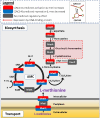
Post-transcriptional gene regulation by non-coding small RNAs (sRNAs) is critical for colonization and survival of enteric pathogens, including the zoonotic pathogen Campylobacter jejuni. In this study, we utilized C. jejuni IA3902 (a representative isolate of the sheep abortion clone) and C. jejuni W7 (a highly motile variant of NCTC 11168, a human gastroenteritis strain) to further investigate regulation by sRNA CjNC110. Both motility and autoagglutination ability were confirmed to be phenotypes of conserved regulation by CjNC110. However, we demonstrated that W7∆CjNC110 does not change chicken colonization levels compared to W7 wild type, directly contrasting IA3902∆CjNC110, which had decreased colonization ability. Subsequently, we determined strain-specific phenotype variation between W7∆CjNC110 and IA3902∆CjNC110 when examining intracellular L-methionine (L-met) levels controlled by the activated methyl cycle (AMC). We hypothesized that the presence of a secondary system for L-met production conferred by MetAB in W7 but not IA3902 might explain the difference in both chicken colonization and L-met availability. Insertion of metAB within IA3902∆CjNC110 (naturally absent) restored intracellular L-met levels in IA3902∆CjNC110::metAB and overcame the colonization defect that resulted from mutagenesis of CjNC110 in IA3902. Deletion of metAB in W7∆CjNC110 (naturally present) led to a decrease in L-met in W7∆CjNC110∆metAB and a colonization defect which was otherwise masked in W7∆CjNC110. Our results indicate that regulation of the AMC leading to altered L-met availability is a conserved regulatory function of CjNC110 in C. jejuni and confirm that L-met generation via the AMC as activated by CjNC110 is critical for optimal host colonization.IMPORTANCEDuring this study, the regulatory action and conservation of function of CjNC110 between two different zoonotically important Campylobacter jejuni strains were examined. Critically, this work for the first time reveals regulation of L-methionine (L-met) production within the activated methyl cycle (AMC) by small RNA (sRNA) CjNC110 as a key factor driving C. jejuni optimal chicken colonization. As a growing body of evidence suggests that maintenance of L-met homeostasis appears to be critical for C. jejuni colonization, interventions targeting the AMC could provide a critical control point for therapeutic drug options to combat this zoonotic pathogen. Our results also indicate that even for conserved sRNAs such as CjNC110, strain-specific differences in phenotypes regulated by sRNAs may exist, independent of conserved regulatory action. Depending on the strain examined and accessory genomic content present, conserved regulatory actions might be masked, thus investigation in multiple strains may be warranted.
Keywords: Campylobacter; L-methionine; activated methyl cycle; chicken colonization; small RNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Role of metAB in Methionine Metabolism and Optimal Chicken Colonization in Campylobacter jejuni.Infect Immun. 2020 Dec 15;89(1):e00542-20. doi: 10.1128/IAI.00542-20. Print 2020 Dec 15. Infect Immun. 2020. PMID: 33046508 Free PMC article.
-
Direct interaction of small non-coding RNAs CjNC140 and CjNC110 optimizes expression of key pathogenic phenotypes of Campylobacter jejuni.mBio. 2023 Aug 31;14(4):e0083323. doi: 10.1128/mbio.00833-23. Epub 2023 Jul 6. mBio. 2023. PMID: 37409826 Free PMC article.
-
Small Noncoding RNA CjNC110 Influences Motility, Autoagglutination, AI-2 Localization, Hydrogen Peroxide Sensitivity, and Chicken Colonization in Campylobacter jejuni.Infect Immun. 2020 Jun 22;88(7):e00245-20. doi: 10.1128/IAI.00245-20. Print 2020 Jun 22. Infect Immun. 2020. PMID: 32366573 Free PMC article.
-
Colonization factors of Campylobacter jejuni in the chicken gut.Vet Res. 2011 Jun 29;42(1):82. doi: 10.1186/1297-9716-42-82. Vet Res. 2011. PMID: 21714866 Free PMC article. Review.
-
A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut.Crit Rev Microbiol. 2012 Feb;38(1):17-29. doi: 10.3109/1040841X.2011.615298. Epub 2011 Oct 13. Crit Rev Microbiol. 2012. PMID: 21995731 Review.
Cited by
-
Transcriptome-wide N6-methyladenosinem modifications analysis of chicken cecum in responding to Campylobacter jejuni inoculation.Front Immunol. 2025 Jul 30;16:1630008. doi: 10.3389/fimmu.2025.1630008. eCollection 2025. Front Immunol. 2025. PMID: 40808945 Free PMC article.
References
-
- Sahin O, Fitzgerald C, Stroika S, Zhao S, Sippy RJ, Kwan P, Plummer PJ, Han J, Yaeger MJ, Zhang Q. 2012. Molecular evidence for zoonotic transmission of an emergent, highly pathogenic Campylobacter jejuni clone in the United States. J Clin Microbiol 50:680–687. doi:10.1128/JCM.06167-11 - DOI - PMC - PubMed
-
- Gaynor EC, Cawthraw S, Manning G, MacKichan JK, Falkow S, Newell DG. 2004. The genome-sequenced variant of Campylobacter jejuni NCTC 11168 and the original clonal clinical isolate differ markedly in colonization, gene expression, and virulence-associated phenotypes. J Bacteriol 186:503–517. doi:10.1128/JB.186.2.503-517.2004 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

