The single-stranded DNA-binding factor SUB1/PC4 alleviates replication stress at telomeres and is a vulnerability of ALT cancer cells
- PMID: 39772744
- PMCID: PMC11745411
- DOI: 10.1073/pnas.2419712122
The single-stranded DNA-binding factor SUB1/PC4 alleviates replication stress at telomeres and is a vulnerability of ALT cancer cells
Abstract
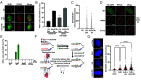
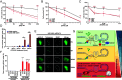
To achieve replicative immortality, cancer cells must activate telomere maintenance mechanisms. In 10 to 15% of cancers, this is enabled by recombination-based alternative lengthening of telomeres pathways (ALT). ALT cells display several hallmarks including heterogeneous telomere length, extrachromosomal telomeric repeats, and ALT-associated PML bodies. ALT cells also have high telomeric replication stress (RS) enhanced by fork-stalling structures (R-loops and G4s) and altered chromatin states. In ALT cells, telomeric RS promotes telomere elongation but above a certain threshold becomes detrimental to cell survival. Manipulating RS at telomeres has thus been proposed as a therapeutic strategy against ALT cancers. Through analysis of genome-wide CRISPR fitness screens, we identified ALT-specific vulnerabilities and describe here our characterization of the roles of SUB1, a ssDNA-binding protein, in telomere stability. SUB1 depletion increases RS at ALT telomeres, profoundly impairing ALT cell growth without impacting telomerase-positive cells. During RS, SUB1 is recruited to stalled forks and ALT telomeres via its ssDNA-binding domain. This recruitment is potentiated by RPA depletion, suggesting that these factors may compete for ssDNA. The viability of ALT cells and their resilience toward RS also requires ssDNA binding by SUB1. SUB1 depletion accelerates cell death induced by FANCM depletion, triggering unsustainable levels of telomeric damage in ALT cells. Finally, combining SUB1 depletion with RS-inducing drugs rapidly induces replication catastrophe in ALT cells. Altogether, our work identifies SUB1 as an ALT susceptibility with roles in the mitigation of RS at ALT telomeres and suggests advanced therapeutic strategies for a host of still poorly managed cancers.
Keywords: DNA damage; DNA replication; alternative lenghtening of telomeres; replication stress; telomeres.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

