The cGAS-STING, p38 MAPK, and p53 pathways link genome instability to accelerated cellular senescence in ATM-deficient murine lung fibroblasts
- PMID: 39772747
- PMCID: PMC11745328
- DOI: 10.1073/pnas.2419196122
The cGAS-STING, p38 MAPK, and p53 pathways link genome instability to accelerated cellular senescence in ATM-deficient murine lung fibroblasts
Abstract
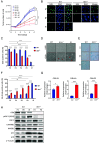
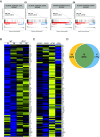
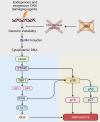
Ataxia-telangiectasia (A-T) is a pleiotropic genome instability syndrome resulting from the loss of the homeostatic protein kinase ATM. The complex phenotype of A-T includes progressive cerebellar degeneration, immunodeficiency, gonadal atrophy, interstitial lung disease, cancer predisposition, endocrine abnormalities, chromosomal instability, radiosensitivity, and segmental premature aging. Cultured skin fibroblasts from A-T patients exhibit premature senescence, highlighting the association between genome instability, cellular senescence, and aging. We found that lung fibroblasts derived from ATM-deficient mice provide a versatile experimental system to explore the mechanisms driving the premature senescence of primary fibroblasts lacking ATM. Atm-/- fibroblasts failed to proliferate under ambient oxygen conditions (21%). Although they initially proliferated under physiological oxygen levels (3%), they rapidly entered senescence. In contrast, wild-type (WT) lung fibroblasts did not senesce under 3% oxygen and eventually underwent immortalization and neoplastic transformation. However, rapid senescence could be induced in WT cells either by Atm gene ablation or persistent chemical inhibition of ATM kinase activity, with senescence induced by ATM inhibition being reversible upon inhibitor removal. Moreover, the concomitant loss of ATM and p53 led to senescence evasion, vigorous growth, rampant genome instability, and subsequent immortalization and transformation. Our findings reveal that the rapid senescence of Atm-/- lung fibroblasts is driven by the collaborative action of the cGAS-STING, p38 MAPK, and p53 pathways in response to persistent DNA damage, ultimately leading to the induction of interferon-α1 and downstream interferon-stimulated genes. We propose that accelerated cellular senescence may exacerbate specific A-T symptoms, particularly contributing to the progressive, life-threatening interstitial lung disease often observed in A-T patients during adulthood.
Keywords: ATM; ataxia–telangiectasia; cGAS-STING; p53; senescence.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- de Almeida L. C., Calil F. A., Machado-Neto J. A., Costa-Lotufo L. V., DNA damaging agents and DNA repair: From carcinogenesis to cancer therapy. Cancer Genet. 252–253, 6–24 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

