Nitrogen source type modulates heat stress response in coral symbiont (Cladocopium goreaui)
- PMID: 39772785
- PMCID: PMC11837503
- DOI: 10.1128/aem.00591-24
Nitrogen source type modulates heat stress response in coral symbiont (Cladocopium goreaui)
Abstract
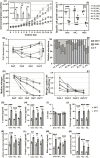
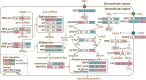
Ocean warming due to climate change endangers coral reefs, and regional nitrogen overloading exacerbates the vulnerability of reef-building corals as the dual stress disrupts coral-Symbiodiniaceae mutualism. Different forms of nitrogen may create different interactive effects with thermal stress, but the underlying mechanisms remain elusive. To address the gap, we measured and compared the physiological and transcriptional responses of the Symbiodiniaceae Cladocopium goreaui to heat stress (31°C) when supplied with different types of nitrogen (nitrate, ammonium, or urea). Under heat stress (HS), cell proliferation and photosynthesis of C. goreaui declined, while cell size, lipid storage, and total antioxidant capacity increased, both to varied extents depending on the nitrogen type. Nitrate-cultured cells exhibited the most robust acclimation to HS, as evidenced by the fewest differentially expressed genes (DEGs) and less ROS accumulation, possibly due to activated nitrate reduction and enhanced ascorbate biogenesis. Ammonium-grown cultures exhibited higher algal proliferation and ROS scavenging capacity due to enhanced carotenoid and ascorbate quenching, but potentially reduced host recognizability due to the downregulation of N-glycan biosynthesis genes. Urea utilization led to the greatest ROS accumulation as genes involved in photorespiration, plant respiratory burst oxidase (RBOH), and protein refolding were markedly upregulated, but the greatest cutdown in photosynthate potentially available to corals as evidenced by photoinhibition and selfish lipid storage, indicating detrimental effects of urea overloading. The differential warming nitrogen-type interactive effects documented here has significant implication in coral-Symbiodiniaceae mutualism, which requires further research.IMPORTANCERegional nitrogen pollution exacerbates coral vulnerability to globally rising sea-surface temperature, with different nitrogen types exerting different interactive effects. How this occurs is poorly understood and understudied. This study explored the underlying mechanism by comparing physiological and transcriptional responses of a coral symbiont to heat stress under different nitrogen supplies (nitrate, ammonium, and urea). The results showed some common, significant responses to heat stress as well as some unique, N-source dependent responses. These findings underscore that nitrogen eutrophication is not all the same, the form of nitrogen pollution should be considered in coral conservation, and special attention should be given to urea pollution.
Keywords: coral reefs; heat stress; nitrogen pollution; symbiotic microalgae; transcriptome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Souter D, Planes S, Wicquart J, Obura D, eds. 2021. Status of coral reefs of the world: 2020. Global Coral Reef Monitoring Network (GCRMN) and International Coral Reef Initiative (ICRI).
-
- Falkowski PG, Dubinsky Z, Muscatine L, Porter JW. 1984. Light and the bioenergetics of a symbiotic coral. Bioscience 34:705–709. doi: 10.2307/1309663 - DOI
-
- Muscatine L, Falkowski PG, Porter JW, Dubinsky Z. 1984. Fate of photosynthetic fixed carbon in light- and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc R Soc Lond B 222:181–202. doi: 10.1098/rspb.1984.0058 - DOI
-
- Muller-Parker G, D’Elia CF, Cook CB. 2015. Interactions between corals and their symbiotic algae, p 99–116. Springer Netherlands.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

