Platelet factor 4-derived C15 peptide broadly inhibits enteroviruses by disrupting viral attachment
- PMID: 39772852
- PMCID: PMC11784221
- DOI: 10.1128/jvi.01859-24
Platelet factor 4-derived C15 peptide broadly inhibits enteroviruses by disrupting viral attachment
Abstract
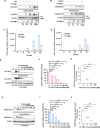
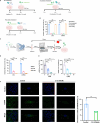
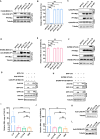
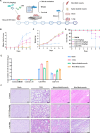
Platelet factor 4 (PF4) has been shown to regulate several viral infections. Our previous study demonstrated that PF4 inhibits the entry of enterovirus A 71 (EV71) and coxsackievirus A16 (CA16), which cause hand, foot, and mouth disease (HFMD). In this study, we report that PF4 also inhibits the circulating HFMD pathogen coxsackievirus A6 (CA6) and the re-emerging enterovirus D68 (EVD68). A 15-amino acid peptide, C15, at the C-terminus of PF4 confers anti-viral activity against multiple enteroviruses (EVs) besides CA6 and EVD68, including EV71 and CA16. Mechanistic studies revealed that wild-type C15 with a net-positive charge (+3), but not its mutants C15M and C15A (both -1), specifically binds to the VP3 capsid protein of CA6 and EVD68, thereby disrupting their attachment to the host cell surface. In addition, VP3 of EVs contains a conserved domain (residues 155-170) crucial for binding to C15. An aspartic acid residue at position 156 imparts a net-negative charge to this domain, which, when substituted with a neutrally charged amino acid, reduces the binding affinity of VP3 for C15. Additionally, C15 protects neonatal mice from lethal challenge upon a CA6 infection. These results suggest that C15 is a promising broad-spectrum anti-viral candidate against multiple EVs.
Importance: EVs, which pose a significant public health threat, can be classified into 15 species, with EV-A, -B, -C, and -D infecting humans and causing a wide range of diseases, from mild illnesses, such as HFMD, to more severe conditions, such as acute flaccid paralysis. The emergence of new and alternative strains highlights the urgent need for broad-spectrum anti-viral agents. In this study, we identified that the C15 of PF4 exhibits potent anti-viral activity against multiple EVs by binding to their surface and blocking their entry into host cells. Furthermore, C15 provides significant protection in vivo. These findings highlight the potential of C15 as a broad-spectrum anti-viral candidate. Our study opens a new avenue for developing treatments to combat the diverse and evolving threats posed by EVs.
Keywords: PF4; broad-spectrum; enterovirus; inhibitor; peptide C15.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Brown DM, Hixon AM, Oldfield LM, Zhang Y, Novotny M, Wang W, Das SR, Shabman RS, Tyler KL, Scheuermann RH. 2018. Contemporary circulating enterovirus D68 strains have acquired the capacity for viral entry and replication in human neuronal cells. mBio 9:e01954-18. doi:10.1128/mBio.01954-18 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81930062, 82272316, 82341072/MOST | National Natural Science Foundation of China (NSFC)
- 2021YFC2301900, 2023YFC2306603, 2301904/MOST | National Key Research and Development Program of China (NKPs)
- YDZJ202201ZYTS521/Science and Technology Department of Jilin Province
- 20102209/Key Laboratory of Molecular Virology, Jilin Province
- 2023588969/Medical Science and Technology Program of Zhejiang Province
LinkOut - more resources
Full Text Sources
Miscellaneous

