The importance of peripheral populations in the face of novel environmental change
- PMID: 39772955
- PMCID: PMC11706656
- DOI: 10.1098/rspb.2024.2331
The importance of peripheral populations in the face of novel environmental change
Abstract
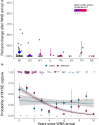
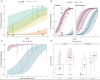
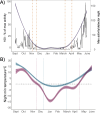
Anthropogenically driven environmental change has imposed substantial threats on biodiversity, including the emergence of infectious diseases that have resulted in declines of wildlife globally. In response to pathogen invasion, maintaining diversity within host populations across heterogenous environments is essential to facilitating species persistence. White-nose syndrome is an emerging fungal pathogen that has caused mass mortalities of hibernating bats across North America. However, in the northeast, peripheral island populations of the endangered northern myotis (Myotis septentrionalis) appear to be persisting despite infection while mainland populations in the core of the species range have experienced sharp declines. Thus, this study investigated host and environmental factors that may contribute to divergent population responses. We compared patterns of pathogen exposure and infection intensity between populations and documented the environmental conditions and host activity patterns that may promote survival despite disease invasion. For island populations, we found lower prevalence and less severe infections, possibly due to a shorter hibernation duration compared to the mainland, which may reduce the time for disease progression. The coastal region of the northern myotis range may serve as habitat refugia that enables this species to persist despite pathogen exposure; however, conservation efforts could be critical to supporting species survival in the long term.
Keywords: host persistence; host–pathogen interactions; northern myotis; peripheral populations; refugia from disease; white-nose snydrome.
Conflict of interest statement
We declare we have no competing interests.
Figures

References
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
