Global research trends on biomarkers for cancer immunotherapy: Visualization and bibliometric analysis
- PMID: 39773010
- PMCID: PMC11730411
- DOI: 10.1080/21645515.2024.2435598
Global research trends on biomarkers for cancer immunotherapy: Visualization and bibliometric analysis
Abstract
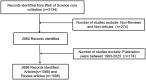
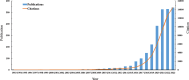
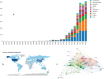
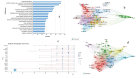
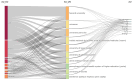
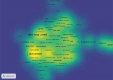
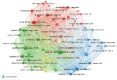
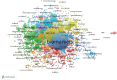
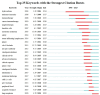
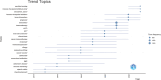
The global burden of cancer continues to grow, posing a significant public health challenge. Although cancer immunotherapy has shown significant efficacy, the response rate is not high. Therefore, the objective of our research was to identify the latest research trends and hotspots on biomarkers from 1993 to 2023. Data were collected from the database Web of Science core collection. Bibliometric analysis and visualization were conducted with CiteSpace(6.3.1), VOSviewer (v1.6.20), R-bibliometrix(v4.3.3), and Microsoft Excel(2019). A total of 2686 literatures were retrieved. The sheer annual volume of publications has shown a rapid upward trend since 2015. The United States has generated the most publications and Harvard University ranked as a leading institution. The global biomarker research on immune checkpoint inhibitors (ICIs) revealed regional differences and in-depth explorations should be promoted in developing countries. Although China has become the second largest country in terms of publication, the average citation per paper and the total link strength were both lower than the other countries. The research on biomarkers mainly concentrated upon the following aspects: PD-1/PD-L1, CTLA-4, gene expression, adverse events, total mutational burden (TMB), body mass index (BMI), gut microbiota, cd8(+)/cd4(+) t-cells, and blood-related biomarkers such as lactate dehydrogenase (LDH), neutrophil-lymphocyte ratio (NLR), cytokines. Furthermore, "artificial intelligence" and "machine learning" have become the most important research hotspot over the last 2 y, which will help us to identify useful biomarkers from complex big data and provide a basis for precise medicine for malignant tumors.
Keywords: Biomarkers; bibliometrics; cancer; immune checkpoint inhibitor; immunotherapy; visualization analysis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
A bibliometric analysis of research on PD-1/PD-L1 in urinary tract tumors.Hum Vaccin Immunother. 2024 Dec 31;20(1):2390727. doi: 10.1080/21645515.2024.2390727. Epub 2024 Oct 10. Hum Vaccin Immunother. 2024. PMID: 39385743 Free PMC article.
-
The research status of immune checkpoint blockade by anti-CTLA4 and anti-PD1/PD-l1 antibodies in tumor immunotherapy in China: A bibliometrics study.Medicine (Baltimore). 2018 Apr;97(15):e0276. doi: 10.1097/MD.0000000000010276. Medicine (Baltimore). 2018. PMID: 29642147 Free PMC article.
-
Insights into the historical trajectory and research trends of immune checkpoint blockade in colorectal cancer: visualization and bibliometric analysis.Front Immunol. 2024 Oct 31;15:1478773. doi: 10.3389/fimmu.2024.1478773. eCollection 2024. Front Immunol. 2024. PMID: 39544944 Free PMC article.
-
PD-1/PD-L1 inhibitors related adverse events: A bibliometric analysis from 2014 to 2024.Hum Vaccin Immunother. 2025 Dec;21(1):2424611. doi: 10.1080/21645515.2024.2424611. Epub 2025 Jan 6. Hum Vaccin Immunother. 2025. PMID: 39757956 Review.
-
A bibliometric study on the impact of gut microbiota on the efficacy of immune checkpoint inhibitors in cancer patients: analysis of the top 100 cited articles.Front Immunol. 2025 Jan 16;15:1519498. doi: 10.3389/fimmu.2024.1519498. eCollection 2024. Front Immunol. 2025. PMID: 39885985 Free PMC article.
References
-
- Pender A, Titmuss E, Pleasance ED, Fan KY, Pearson H, Brown SD, Grisdale CJ, Topham JT, Shen Y, Bonakdar M, et al. Genome and transcriptome biomarkers of response to immune checkpoint inhibitors in advanced solid tumors. Clin Cancer Res. 2021;27(1):202–212. doi:10.1158/1078-0432.CCR-20-1163. - DOI - PubMed
-
- Geoerger B, Kang HJ, Yalon-Oren M, Marshall LV, Vezina C, Pappo A, Laetsch TW, Petrilli AS, Ebinger M, Toporski J, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020;21(1):121–133. doi:10.1016/S1470-2045(19)30671-0. - DOI - PubMed
-
- Davis KL, Fox E, Merchant MS, Reid JM, Kudgus RA, Liu X, Minard CG, Voss S, Berg SL, Weigel BJ, et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020;21(4):541–550. doi:10.1016/S1470-2045(20)30023-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
