Prediction of post-insertion infections related to totally implantable subcutaneous venous access ports in tumor patients using a nomogram
- PMID: 39773012
- PMCID: PMC12097388
- DOI: 10.17305/bb.2024.11583
Prediction of post-insertion infections related to totally implantable subcutaneous venous access ports in tumor patients using a nomogram
Abstract
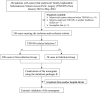
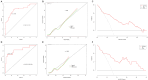
Totally implantable subcutaneous venous access ports (TISVAPs) are essential for long-term central venous chemotherapy, delivering medication directly into the central veins of patients. While they play a critical role in reducing patient discomfort, TISVAPs pose a notable risk of post-insertion infections-particularly concerning for oncology patients with compromised immune systems due to aggressive treatment regimens. Our research addresses this issue by developing a predictive nomogram to estimate the risk of TISVAP-associated infections. The model is based on independent risk factors identified in our study: a history of diabetes, the type of chemotherapy, peripheral blood leukocyte count (WBC), and serum albumin levels. Using retrospective clinical data from 309 oncology patients who underwent TISVAP implantation at a tertiary A-grade comprehensive hospital, we divided the dataset into training (n = 246) and validation (n = 63) subsets. Through logistic and Lasso regression analyses, we identified the independent risk factors associated with infections. The resulting interactive nomogram demonstrated strong accuracy and reliability, with C-indexes of 0.82 and 0.835 for the training and validation sets, respectively. This tool equips healthcare providers to proactively identify high-risk patients and tailor preventive strategies accordingly. Ultimately, our research aims to enhance patient outcomes and improve the quality of life for those undergoing long-term venous chemotherapy.
Conflict of interest statement
Conflicts of interest: Authors declare no conflicts of interest.
Figures


References
-
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–71. https://doi.org/10.4065/81.9.1159. - PubMed
-
- Blot F, Laplanche A, Raynard B, Germann N, Antoun S, Nitenberg G. Accuracy of totally implanted ports, tunnelled, single- and multiple-lumen central venous catheters for measurement of central venous pressure. Intensive Care Med. 2000;26(12):1837–42. https://doi.org/10.1007/s001340000705. - PubMed
-
- Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA Cancer J Clin. 2008;58(6):323–46. https://doi.org/10.3322/CA.2008.0015. - PubMed
-
- Lebeaux D, Fernández-Hidalgo N, Chauhan A, Lee S, Ghigo JM, Almirante B, et al. Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis. 2014;14(2):146–59. https://doi.org/10.1016/S1473-3099(13)70266-4. - PubMed
-
- Tabatabaie O, Kasumova GG, Eskander MF, Critchlow JF, Tawa NE, Tseng JF. Totally implantable venous access devices: a review of complications and management strategies. Am J Clin Oncol. 2017;40(1):94–105. https://doi.org/10.1097/COC.0000000000000361. - PubMed

