Engaging dystonia networks with subthalamic stimulation
- PMID: 39773021
- PMCID: PMC11745339
- DOI: 10.1073/pnas.2417617122
Engaging dystonia networks with subthalamic stimulation
Abstract
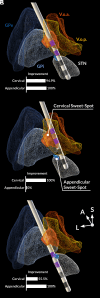
Deep brain stimulation is an efficacious treatment for dystonia. While the internal pallidum serves as the primary target, recently, stimulation of the subthalamic nucleus (STN) has been investigated. However, optimal targeting within this structure and its surroundings have not been studied in depth. Indeed, historical targets that have been used for surgical treatment of dystonia are directly adjacent to the STN. Further, multiple types of dystonia exist, and outcomes are variable, suggesting that not all types would profit maximally from the same target. Therefore, a thorough investigation of neural substrates underlying stimulation effects on dystonia signs and symptoms is warranted. Here, we analyze a multicenter cohort of isolated dystonia patients with subthalamic implantations (N = 58) and relate their stimulation sites to improvements of appendicular and cervical symptoms as well as blepharospasm. Stimulation of the ventral oral posterior nucleus of thalamus and surrounding regions were associated with improvements in cervical dystonia, while stimulation of the dorsolateral STN was associated with improvements in limb dystonia and blepharospasm. This dissociation was matched by structural connectivity analysis, where the cerebellothalamic, corticospinal, and pallidosubthalamic tracts were associated with improvements of cervical dystonia, while hyperdirect and subthalamopallidal pathways with alleviation of limb dystonia and blepharospasm. On the level of functional networks, improvements of limb dystonia were associated with connectivity to the corresponding somatotopic regions in the primary motor cortex, while alleviation of cervical dystonia to the cingulo-opercular network. These findings shed light on the pathophysiology of dystonia and may guide DBS targeting and programming in the future.
Keywords: cervical dystonia; deep brain stimulation; limb dystonia; structural connectivity; sweet-spot analysis.
Conflict of interest statement
Competing interests statement:R.L. reports personal fees from Medtronic. M.D.F. has intellectual property on the use of brain connectivity imaging to analyze lesions and guide brain stimulation, is a consultant for Magnus Medical, Soterix, Abbott, and Boston Scientific, and has received research funding from Neuronetics. A.H. reports lecture fees for Boston Scientific and is a consultant for FxNeuromodulation and Abbott. K.B., C.N., T.A.D., B.H., G.M.M., N.L., S.O., B.H.B., B.A.-F., E.M.G., N.U.F.D., C.G., M.H., H.A.J., P.A.S., J.L.O., Y.W., and C.Z. declare no competing interests.
Figures
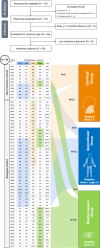
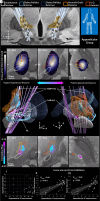
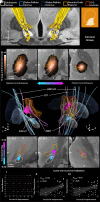
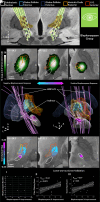

Update of
-
Engaging dystonia networks with subthalamic stimulation.medRxiv [Preprint]. 2024 May 25:2024.05.24.24307896. doi: 10.1101/2024.05.24.24307896. medRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Jan 14;122(2):e2417617122. doi: 10.1073/pnas.2417617122. PMID: 38903109 Free PMC article. Updated. Preprint.
Similar articles
-
Engaging dystonia networks with subthalamic stimulation.medRxiv [Preprint]. 2024 May 25:2024.05.24.24307896. doi: 10.1101/2024.05.24.24307896. medRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Jan 14;122(2):e2417617122. doi: 10.1073/pnas.2417617122. PMID: 38903109 Free PMC article. Updated. Preprint.
-
Modulation of Cerebellar Oscillations with Subthalamic Stimulation in Patients with Parkinson's Disease.J Parkinsons Dis. 2024;14(7):1417-1426. doi: 10.3233/JPD-240065. J Parkinsons Dis. 2024. PMID: 39331106 Free PMC article.
-
Subthalamic and pallidal oscillations and their couplings reflect dystonia severity and improvements by deep brain stimulation.Neurobiol Dis. 2024 Sep;199:106581. doi: 10.1016/j.nbd.2024.106581. Epub 2024 Jun 25. Neurobiol Dis. 2024. PMID: 38936434
-
Botulinum toxin type B for cervical dystonia.Cochrane Database Syst Rev. 2005 Jan 25;(1):CD004315. doi: 10.1002/14651858.CD004315.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2016 May 13;(5):CD004315. doi: 10.1002/14651858.CD004315.pub3. PMID: 15674941 Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Efficacy and safety of multiple-target deep brain stimulation in non-parkinsonian movement disorders: a systematic review.Neurosurg Rev. 2025 Jun 17;48(1):512. doi: 10.1007/s10143-025-03661-4. Neurosurg Rev. 2025. PMID: 40526321
References
-
- Coubes P., et al. , Treatment of early-onset generalized dystonia by chronic bilateral stimulation of the internal globus pallidus. Apropos of a case. Neurochirurgie 45, 139–144 (1999). - PubMed
-
- Krauss J. K., Pohle T., Weber S., Ozdoba C., Burgunder J. M., Bilateral stimulation of globus pallidus internus for treatment of cervical dystonia. Lancet Lond. Engl. 354, 837–838 (1999). - PubMed
-
- Kupsch A., et al. , Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N. Engl. J. Med. 355, 1978–1990 (2006). - PubMed
-
- Volkmann J., et al. , Pallidal neurostimulation in patients with medication-refractory cervical dystonia: A randomised, sham-controlled trial. Lancet Neurol. 13, 875–884 (2014). - PubMed
Publication types
MeSH terms
Grants and funding
- 2R01 MH113929/Foundation for the National Institutes of Health (FNIH)
- 1R01NS127892-01/Foundation for the National Institutes of Health (FNIH)
- R01 NS127892/NS/NINDS NIH HHS/United States
- R01 MH113929/MH/NIMH NIH HHS/United States
- R01 MH130666/MH/NIMH NIH HHS/United States
- R01 13478451/Foundation for the National Institutes of Health (FNIH)
- R21 MH126271/MH/NIMH NIH HHS/United States
- FFOR Seed Grant/New Venture Fund (NVF)
- R21 NS123813/NS/NINDS NIH HHS/United States
- UM1NS132358/Foundation for the National Institutes of Health (FNIH)
- UM1 NS132358/NS/NINDS NIH HHS/United States
- R56 AG069086/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

