Epistatic hotspots organize antibody fitness landscape and boost evolvability
- PMID: 39773024
- PMCID: PMC11745389
- DOI: 10.1073/pnas.2413884122
Epistatic hotspots organize antibody fitness landscape and boost evolvability
Abstract
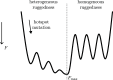
The course of evolution is strongly shaped by interaction between mutations. Such epistasis can yield rugged sequence-function maps and constrain the availability of adaptive paths. While theoretical intuition is often built on global statistics of large, homogeneous model landscapes, mutagenesis measurements necessarily probe a limited neighborhood of a reference genotype. It is unclear to what extent local topography of a real epistatic landscape represents its global shape. Here, we demonstrate that epistatic landscapes can be heterogeneously rugged and this heterogeneity may render biomolecules more evolvable. By characterizing a multipeaked fitness landscape of a SARS-CoV-2 antibody mutant library, we show that heterogeneous ruggedness arises from sparse epistatic hotspots, whose mutation impacts the fitness effect of numerous sequence sites. Surprisingly, mutating an epistatic hotspot may enhance, rather than reduce, the accessibility of the fittest genotype, while increasing the overall ruggedness. Further, migratory constraints in real space alleviate mutational constraints in sequence space, which not only diversify direct paths taken but may also turn a road-blocking fitness peak into a stepping stone leading toward the global optimum. Our results suggest that a hierarchy of epistatic hotspots may organize the fitness landscape in such a way that path-orienting ruggedness confers global smoothness.
Keywords: combinatorial mutagenesis; epistasis; evolvability; heterogeneity; sequence–function map.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures





Similar articles
-
Ensemble-Based Mutational Profiling and Network Analysis of the SARS-CoV-2 Spike Omicron XBB Lineages for Interactions with the ACE2 Receptor and Antibodies: Cooperation of Binding Hotspots in Mediating Epistatic Couplings Underlies Binding Mechanism and Immune Escape.Int J Mol Sci. 2024 Apr 12;25(8):4281. doi: 10.3390/ijms25084281. Int J Mol Sci. 2024. PMID: 38673865 Free PMC article.
-
Physical Constraints on Epistasis.Mol Biol Evol. 2020 Oct 1;37(10):2865-2874. doi: 10.1093/molbev/msaa124. Mol Biol Evol. 2020. PMID: 32421772
-
Genetic complementation fosters evolvability in complex fitness landscapes.Sci Rep. 2023 Jan 12;13(1):662. doi: 10.1038/s41598-022-26588-y. Sci Rep. 2023. PMID: 36635310 Free PMC article.
-
Global epistasis on fitness landscapes.Philos Trans R Soc Lond B Biol Sci. 2023 May 22;378(1877):20220053. doi: 10.1098/rstb.2022.0053. Epub 2023 Apr 3. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37004717 Free PMC article. Review.
-
Rational evolutionary design: the theory of in vitro protein evolution.Adv Protein Chem. 2000;55:79-160. doi: 10.1016/s0065-3233(01)55003-2. Adv Protein Chem. 2000. PMID: 11050933 Review.
Cited by
-
Convergent and clonotype-enriched mutations in the light chain drive affinity maturation of a public antibody.bioRxiv [Preprint]. 2025 Mar 10:2025.03.07.642041. doi: 10.1101/2025.03.07.642041. bioRxiv. 2025. Update in: Cell Rep. 2025 Aug 26;44(8):116122. doi: 10.1016/j.celrep.2025.116122. PMID: 40161664 Free PMC article. Updated. Preprint.
References
-
- De Visser J. A. G., Krug J., Empirical fitness landscapes and the predictability of evolution. Nat. Rev. Genet. 15, 480–490 (2014). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

