Antibody-based delivery of interleukin-2 modulates the immunosuppressive tumor microenvironment and achieves cure in pancreatic ductal adenocarcinoma syngeneic mice
- PMID: 39773310
- PMCID: PMC11705946
- DOI: 10.1186/s13046-024-03238-x
Antibody-based delivery of interleukin-2 modulates the immunosuppressive tumor microenvironment and achieves cure in pancreatic ductal adenocarcinoma syngeneic mice
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and deadly type of cancer, with an extremely low five-year overall survival rate. To date, current treatment options primarily involve various chemotherapies, which often prove ineffective and are associated with substantial toxicity. Furthermore, immunotherapies utilizing checkpoint inhibitors have shown limited efficacy in this context, highlighting an urgent need for novel therapeutic strategies. This study investigates the preclinical efficacy of an innovative targeted therapy based on antibody-cytokine fusion proteins, specifically interleukin-2 (IL-2), a pivotal driver of cell-mediated immunity, fused to L19 antibody, which selectively binds to extra domain B of fibronectin (EDB-FN1) expressed in the tumor microenvironment.
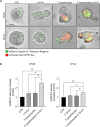
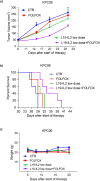
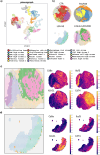
Methods: We tested the effectiveness of different immunocytokines through in vivo characterization in syngeneic C57BL/6J orthotopic mouse models of PDAC. Based on these results, we decided to focus on L19-IL2. To assess the efficacy of this immunocytokine we developed an ex-vivo immune-spheroid interaction platform derived from murine 3D pancreatic cultures, and telomerase reverse transcriptase (TERT) specific T-lymphocytes. Moreover, we evaluated the anti-cancer effect of L19-IL2 in combination with standard therapy in vivo experiments in PDAC mouse models. Tumor samples collected after the treatments were characterized for tumor infiltrating immune cell components by bulk RNA sequencing (RNA-seq) and spatial transcriptomics (Stereo-seq) analysis.
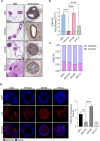
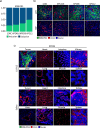
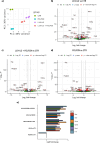
Results: The tumor-targeted L19-IL2 fusion protein demonstrated potent, dose-dependent anti-tumor activity in mice with pancreatic tumors resistant to standard chemotherapy. Spatial Transcriptomics (ST) and RNA-seq analyses indicated that L19-IL2 treatment induced a significant influx of immune cells into the tumor microenvironment, with these cells expressing activation markers like granzymes, perforins, and the IL-2 receptors.
Conclusions: Our results demonstrated that L19-IL2 enhances immune infiltration and cytotoxicity, remodeling the "cold" tumor microenvironment (TME) in PDAC. This innovative antibody-cytokine fusion protein improves therapeutic outcomes, paving the way for novel targeted treatment strategies in PDAC.
Keywords: Chemotherapy; Immunocytokines; Orthotopic syngeneic mouse models; Pancreatic cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Animal experiments were approved by animal ethics committee of Catholic University of the Sacred Heart of Rome and by Ministry of Health with approval number n°593/2019-PR. This study was approved by the ethics committee of AOUI Verona Hospital (Territorial Ethics Committee for the South-West Veneto Area) with approval number Prot. n°53732. The patients provided written consent. This study was carried out in accordance with Declaration of Helsinki. Consent for publication: All patients provided consent for publication. Competing interests: Dario Neri is a co-founder and shareholder of Philogen ( www.philogen.com ), a Swiss-Italian Biotech company that operates in the field of ligand-based pharmacodelivery. Roberto De Luca, and Emanuele Puca are employees of Philochem AG, a daughter company of Philogen acting as discovery unit of the group.
Figures
References
-
- Jiang Y, Sohal DPS. Pancreatic adenocarcinoma management. JCO Oncol Pract. 2023;19(1):19–32. - PubMed
-
- Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2(1):16022. - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

