Metformin reverts aortic calcifications and elastin loss induced by an experimental metabolic syndrome
- PMID: 39773346
- PMCID: PMC11770402
- DOI: 10.1530/EC-24-0714
Metformin reverts aortic calcifications and elastin loss induced by an experimental metabolic syndrome
Abstract
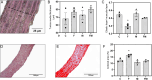
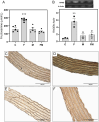
Metabolic syndrome (MetS) is associated with osteogenic transdifferentiation of vascular smooth muscle cells (VSMCs) and accumulation of arterial calcifications (ACs). Metformin (MET) inhibits this transdifferentiation in vitro. Here, we evaluate the in vivo efficacy of oral MET to reduce AC in a model of MetS. Twenty young male Wistar rats were divided into two groups: one received water and the other received water plus 20% fructose to induce MetS. After 14 days, and for another 4 weeks, MET (100 mg/kg per day) was added to half of each group's drinking source, thus C (water), F (fructose), M (MET) and FM (fructose + MET). Serum and adipose tissue were collected. Aortas were dissected for histomorphometric and immunohistochemical analysis, ex vivo calcification studies and isolation of VSMCs to measure their alkaline phosphatase activity (ALP), collagen production, extracellular mineralization, gene expression of RUNX2 and receptor for advanced glycation end-products (AGEs) (RAGE), and elastic fiber production. F group showed parameters compatible with MetS. Aortic tunica media from F showed decreased elastic-to-muscular layer ratio, increased collagen content and increased levels of the AGEs structure carboxymethyl-lysine. Aortic arches from F presented a tendency for higher ex vivo calcification. VSMCs from F showed increased ALP, collagen secretion, mineralization and expression of RUNX2 and RAGE, and decreased elastic fiber production. All these effects were reverted by MET cotreatment (FM group). In vitro, AGEs-modified bovine serum albumin upregulated RAGE expression of control VSMCs, and this was prevented by MET in an AMP kinase-dependent manner. Thus, experimental MetS induces RAGE upregulation and osteogenic transdifferentiation of aortic VSMCs curbed by oral treatment with MET.
Keywords: advanced glycation end-products; fructose; metabolic syndrome; metformin; osteogenic transdifferentiation; receptor for advanced glycation end-products; vascular smooth muscle cells.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the work reported. Dr Antonio McCarthy is a Senior Editor of
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

