Thirty years of StAR gazing. Expanding the universe of the steroidogenic acute regulatory protein
- PMID: 39773408
- PMCID: PMC11840834
- DOI: 10.1530/JOE-24-0310
Thirty years of StAR gazing. Expanding the universe of the steroidogenic acute regulatory protein
Abstract
The current understanding of the biology, biochemistry and genetics of the steroidogenic acute regulatory protein (StAR) and its deficiency state (lipoid congenital adrenal hyperplasia, lipoid CAH) involves the complex interplay of four areas of study: the acute regulation of steroidogenesis, clinical phenomena in lipoid CAH, the enzymatic conversion of cholesterol to pregnenolone in steroidogenic mitochondria, and the cell biology of StAR. This review traces the origins of these areas of study, describes how they have been woven into an increasingly coherent fabric and tries to explore some remaining loose ends in this ongoing field of endocrine research. Extensive research from multiple laboratories has established that StAR is required for the rapid, abundant steroidal responses of the adrenals and gonads, but all steroidogenic cells, especially the placenta, also have StAR-independent steroidogenesis, whose basis remains under investigation. Lipoid CAH is the StAR knockout of nature whose complex (and unexpected) clinical features are explained by the 'two-hit model', in which StAR-dependent steroidogenesis and StAR-independent steroidogenesis are lost sequentially. StAR is targeted to mitochondria and acts on the outer mitochondrial membrane before being imported via the 'translocase of outer membrane' system and is then inactivated by mitochondrial proteases. A role for the 'translocator protein' (TSPO) has long been proposed, but an essential role for TSPO is excluded by recent transgenic mouse experiments. Crystal structures show that a StAR molecule can bind one cholesterol but does not explain how each StAR molecule triggers the import of hundreds of cholesterol molecules; this is the most pressing area for future research.
Keywords: acute response; cholesterol; congenital lipoid adrenal hyperplasia; mitochondria; steroid.
Conflict of interest statement
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of this work.
Figures
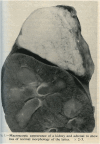

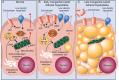
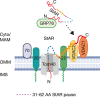
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

