Safety and efficacy of the therapeutic DNA-based vaccine VB10.16 in combination with atezolizumab in persistent, recurrent or metastatic HPV16-positive cervical cancer: a multicenter, single-arm phase 2a study
- PMID: 39773564
- PMCID: PMC11749841
- DOI: 10.1136/jitc-2024-010827
Safety and efficacy of the therapeutic DNA-based vaccine VB10.16 in combination with atezolizumab in persistent, recurrent or metastatic HPV16-positive cervical cancer: a multicenter, single-arm phase 2a study
Abstract
Background: Second-line treatment options for persistent, recurrent or metastatic (r/m) cervical cancer are limited. We investigated the safety, efficacy, and immunogenicity of the therapeutic DNA-based vaccine VB10.16 combined with the immune checkpoint inhibitor atezolizumab in patients with human papillomavirus (HPV)16-positive r/m cervical cancer.
Patients and methods: This multicenter, single-arm, phase 2a study (NCT04405349, registered 26 May 2020) enrolled adult patients with persistent, r/m HPV16-positive cervical cancer. Patients received 3 mg VB10.16 (every 3 weeks (Q3W) for 12 weeks, hereafter every 6 weeks) combined with 1,200 mg atezolizumab (Q3W) for 48 weeks in total with a 12-month follow-up. The primary endpoints were incidence and severity of adverse events (AEs) and objective response rate (ORR; Response Evaluation Criteria in Solid Tumor V.1.1). ORR was assessed in the efficacy population, being all response-evaluable patients who received any administration of VB10.16 and atezolizumab and had at least one post-baseline imaging assessment.
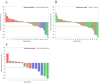
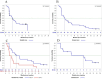
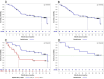
Results: Between June 16, 2020, and January 25, 2022, 52 patients received at least one administration of study treatment. Of these, 47 patients had a minimum of one post-baseline tumor assessment. The median follow-up time for survival was 11.7 months. AEs related to VB10.16 were non-serious and mainly mild injection site reactions (9 of 52 patients). There were no signs of new toxicities other than what was already described with atezolizumab. ORR was 19.1% (95% CI 9.1% to 33.3%). Median duration of response was not reached (n.r.) (95% CI 2.2 to n.r.), median progression-free survival was 4.1 months (95% CI 2.1 to 6.2), and median overall survival was 21.3 months (95% CI 8.5 to n.r.). In programmed death-ligand 1 (PD-L1)-positive patients (n=24), ORR was 29.2% (95% CI 12.6 to 51.1). HPV16-specific T-cell responses were analyzed in 36 of 47 patients with an increase observed in 22/36 (61%).
Conclusions: The therapeutic DNA-based vaccine VB10.16 combined with atezolizumab was safe and well tolerated showing a promising clinically meaningful efficacy with durable responses in patients with persistent, r/m HPV16-positive cervical cancer, especially if PD-L1-positive.
Keywords: Cervical Cancer; Immunotherapy.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: PH, MZ, FF, LW, HGD, MB, J-FB, PB, LR, JC, FM, CD, TL, and KL received funding to their institutions from Nykode Therapeutics to support the study. PH reports support for attending meeting and/or travel from Nykode Therapeutics. LW reports consulting fees from MSD and Seagen; payment or honoraria from Seagen, MSD, Roche, Novartis, Eisai, GSK, Pfizer, and Medupdate GmbH; participation on Data Safety Monitoring Board or Advisory Board for Seagen, MSD, Roche, Eisai and GSK; ESCVD president and AGO study group executive board member. HGD reports institutional consulting fees from GSK, MSD and AstraZeneca; institutional payment or honoraria from AstraZeneca, MSD and Roche; institutional support for attending meeting and/or travel from MSD, AstraZeneca and Roche; participation on Data Safety Monitoring Board or Advisory Board (institutional) for MSD and AstraZeneca. MB reports consulting fees from AstraZeneca and GSK for participation on Advisory Board; payment or honoraria from MSD for lecture. FM reports institutional and/or personal consulting fees from AstraZeneca, Eisai, Daiichi Sankyo, Genomic Health, GSK, Immuncom, Gilead, Seagen, MSD, Myriad, Novartis, PharmaMar, Roche, Stemline Menarini, Nykode Therapeutics, and Onkowissen.TV; institutional and personal payment or honoraria from AstraZeneca, Clovis, Daiichi Sankyo, GSK, Lilly, MSD, Myriad, Stemline Menarini, Roche, Gilead, Seagen, and Pfizer; support for attending meeting and/ travel from AstraZeneca, Roche, Stemline, Pfizer, Gilead, Daiichi Sankyo, GSK, and MSD; Participation on Safety Monitoring Board or Advisory Board for Immutep and Palleos_Phaon. CD reports consulting fee from MSD; payment or honoraria for lectures from Lilly, MSD, GSK, AstraZeneca and Eisai. TL reports consulting fees from MSD and Roche; payment or honoraria from Roche, Novartis, Amgen, GSK, Pfizer, Gilead, MSD, Lilly, AstraZeneca, Daiichi, and Sankyo; support for attending meetings and/or travel from Daiichi, Sankyo, Pfizer, Gilead, AstraZeneca, and Stemline; participation in Advisory Board for GSK, Gilead, Roche, Pfizer, Daiichi, Sankyo, MSD, and Lilly. KL reports research funding from GSK; consulting fees for Advisory boards from AstraZeneca, MSD, Nykode Therapeutics, Eisai and GSK; participation on Safety Monitoring Board for Karyopharm; Deputy Medical Director in Nordic Society of Gynaecological Trial Unit until 2023. MZ, FF, J-FB, PB, LR and JC declare no conflicts of interest.AR, KCGB, and RSO are employees of Nykode Therapeutics, and RSO hold stocks or stock options in the company. RSO also holds stocks or stock options in Genmab, Novo Nordisk, H. Lundbeck and Y-mAbs Therapeutics.
Figures
References
-
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) The Lancet. 2017;390:1654–63. doi: 10.1016/S0140-6736(17)31607-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
