Disruption of tumor-intrinsic PGAM5 increases anti-PD-1 efficacy through the CCL2 signaling pathway
- PMID: 39773565
- PMCID: PMC11749670
- DOI: 10.1136/jitc-2024-009993
Disruption of tumor-intrinsic PGAM5 increases anti-PD-1 efficacy through the CCL2 signaling pathway
Abstract
Background: Immunosuppressive phenotype compromised immunotherapy efficacy of hepatocellular carcinoma. Tumor cells intrinsic mitochondria dynamics could pass effects on the extracellular microenvironment through mtDNA stress. PGAM5 anchors at mitochondria and regulates mitochondria functions. We aim to explore whether the regulation of tumor-intrinsic PGAM5 on mitochondria affects tumor-infiltrating immune cells in the microenvironment and whether tumor-intrinsic PGAM5 can be a therapeutic target to enhance the immunotherapy efficacy of hepatocellular carcinoma (HCC).
Methods: We analyzed the correlation of PGAM5 expression and immune cells infiltration using Gene Expression Omnibus (GEO) and The Cancer Genome Atlas Liver Hepatocellular Carcinoma (TCGA-LIHC) data sets based on cibersort algorithm and tumor-tissue arrays from two independent cohorts. To further validate our findings, we established subcutaneous and orthotopic mouse HCC models with tumor-intrinsic Pgam5 deficiency and analyzed tumor-infiltrating immune cells by flow cytometry and single-cell RNA sequencing. Mechanistically, we established an in vitro co-culture system and analyzed proteomics data to find out the bridge between tumor cell PGAM5 and tumor-associated macrophages (TAMs) in the microenvironment. Immunofluorescence, chromatin-immunoprecipitation, ELISA, mass spectrometry were conducted to explore the molecular pathway. Macrophages were depleted to investigate whether the effects of tumor-intrinsic PGAM5 on TAMs could affect immunotherapy efficacy in HCC orthotopic and subcutaneous mouse models.
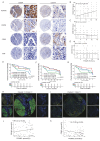
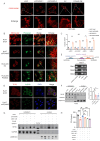
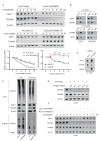
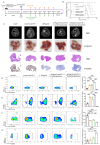
Results: PGAM5 expression in tumor was positively correlated with M2-phenotype TAM infiltration in patients with both HCC and mouse HCC tumor models. High tumor-intrinsic PGAM5 expression promoting M2 TAMs infiltration correlated with poor clinical-pathological characteristics and prognosis in patients with HCC. Disruption of tumor-intrinsic Pgam5 reduced TAM M2 polarization and inhibited HCC tumor growth in tumor-bearing mice. Mechanistically, in HCC cells PGAM5 deficiency inhibited mitochondria fission by promoting TRIM28 binding with DRP1, which increased ubiquitination and degradation of DRP1. Tumor-intrinsic PGAM5 deficiency mediated mitochondria fusion and reduced cytosolic mtDNA stress which attenuated TLR9 activation and downstream NF-κB-regulated CCL2 secretion. Furthermore, disruption of tumor-intrinsic Pgam5 significantly facilitated CD8+ T cells activation and improved anti-programmed cell death protein-1 therapeutic efficacy with macrophages depletion compromising synergistic antitumor immune response.
Conclusion: Our results shed light on the effect of tumor mitochondria dynamics on TAMs in tumor microenvironment. Tumor-intrinsic PGAM5 can be a therapeutic target to improve immunotherapy efficacy in patients with HCC.
Keywords: Cytokine; Hepatocellular Carcinoma; Immune modulatory; Macrophage; Mitochondria.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
