Targeting the NLRP3 inflammasome abrogates cardiotoxicity of immune checkpoint blockers
- PMID: 39773567
- PMCID: PMC11749606
- DOI: 10.1136/jitc-2024-010127
Targeting the NLRP3 inflammasome abrogates cardiotoxicity of immune checkpoint blockers
Abstract
Background: Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of many malignant tumors. However, ICI-induced hyper-immune activation causes cardiotoxicity. Traditional treatments such as glucocorticoids and immunosuppressants have limited effectiveness and may even accelerate tumor growth. This study aimed to identify approaches that effectively reduce cardiotoxicity and simultaneously preserve or enhance the antitumor immunity of ICI therapy.
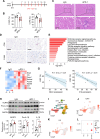
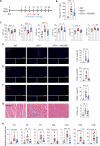
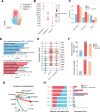
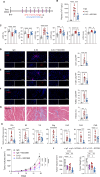
Methods: ICI injection in melanoma-bearing C57BL/6J female mice was used to simulate cardiotoxicity in patients with tumor undergoing immune therapy. MCC950 was used to block nod-like receptor protein 3 (NLRP3) inflammasome activity. Echocardiography, immunofluorescence, flow cytometry, and reverse transcription quantitative polymerase chain reaction were used to assess cardiac function, immune cell populations, and inflammatory factor levels. Bulk and single-cell RNA sequencing was used to detect the changes in cardiac transcriptome and immunological network.
Results: NLRP3 inhibition reduced inflammatory response and improved cardiac function. Notably, NLRP3 inhibition also resulted in a pronounced suppression of tumor growth. Single-cell RNA sequencing elucidated that MCC950 treatment reduced the cardiac infiltration of pathogenic macrophages, cytotoxic T cells, activated T cells, and their production of inflammatory cytokines, while enhancing the presence of reparative macrophages and naive T cells. In addition, MCC950 attenuated cardiotoxicity induced by dual programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) immunotherapy and promoted tumor regression, and showed efficacy in treating established cardiotoxicity.
Conclusions: Our findings provide a promising clinical approach for preventing and treating cardiotoxicity induced by ICIs, dissociating the antitumor efficacy of ICI-based therapies from their cardiotoxic side effects.
Keywords: Cardiotoxicity; Immune Checkpoint Inhibitor; Immunotherapy; Macrophage; T cell.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
