Field study on the suitability of oral fluid samples for monitoring of Lawsonia intracellularis and Brachyspira hyodysenteriae by multiplex qPCR under field conditions
- PMID: 39773664
- PMCID: PMC11706030
- DOI: 10.1186/s40813-024-00415-4
Field study on the suitability of oral fluid samples for monitoring of Lawsonia intracellularis and Brachyspira hyodysenteriae by multiplex qPCR under field conditions
Abstract
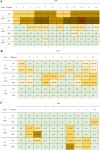
Background: Monitoring or surveillance of infectious diseases is crucial in terms of herd health management of livestock. Investigations of oral fluids have become an animal friendly routine strategy to monitor respiratory pathogens in pigs. Less is known about the suitability of oral fluids for the detection of enteric pathogens in swine. In the present study we evaluated the use of oral fluids to monitor B. hyodysenteriae and L. intracellularis compared to pooled fecal samples by multiplex qPCR in a pen-wise follow-up of fattening pigs. Therefore, we collected oral fluids at an age of 12, 16 and 20 weeks of life and compared them to pooled fecal samples collected from the same pens on two fattening farms.
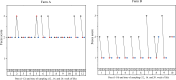
Results: Cohen´s Kappa analysis revealed a substantial agreement between oral fluids and pooled fecal samples on pen level (Cohen´s Kappa: 0.745; p < 0.001). DNA-loads of L. intracellularis were tendentially higher (p = 0.053) in pooled fecal samples than in the corresponding OFs.
Conclusions: The present study shows that oral fluids are an appropriate tool to monitor B. hyodysenteriae and L. intracellularis on conventional fattening farms under field conditions. However, multiple pen testing should be conducted to increase the diagnostic performance and sensitivity.
Keywords: Enteric diseases; Fattening; Livestock; Screening; Swine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval was waived as oral fluid sampling is not rated as animal testing by the appropriate authority (Az. 2532.Vet_03-18–43). Consent for publication: Written informed consent was obtained from the owners of the animals. Competing interests: The authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources

