Validation of biomarkers and clinical scores for the detection of uterine leiomyosarcoma: a case-control study with an update of pLMS
- PMID: 39773707
- PMCID: PMC11708173
- DOI: 10.1186/s12885-024-13396-y
Validation of biomarkers and clinical scores for the detection of uterine leiomyosarcoma: a case-control study with an update of pLMS
Abstract
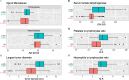
Background: The diagnosis of rare uterine leiomyosarcoma (uLMS) remains a challenge given the high incidence rates of benign uterine tumors such as leiomyoma (LM). In the last decade, several clinical scores and blood serum markers have been proposed. The aim of this study is to validate and update the pLMS clinical scoring system, evaluating the accuracy of the scoring system by Zhang et al. and examining the discriminatory ability of blood markers such as serum lactate dehydrogenase (LDH), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR).
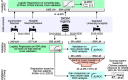
Methods: In a case-control study, 90 new uLMS from the DKSM consultation registry and 659 prospectively recruited LM cases from the Saarland University Hospital were used for validation. Welch's t-test and Hedges' g were used to evaluate blood markers and optimal thresholds and diagnostic odds ratios were calculated. Scoring systems were compared using receiver operating characteristics and proposed diagnostic cut-offs were reviewed. Missing values were imputed by random forest imputation to create the updated scoring system 'pLMS2' using penalized logistic regression based on the pooled data sets of 384 uLMS and 1485 LM.
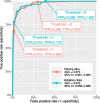
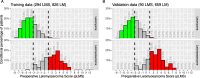
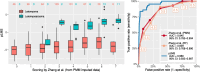
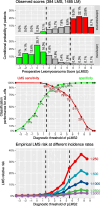
Results: pLMS achieved an AUC of 0.97 on the validation data, but sensitivity and specificity varied at the proposed thresholds due to a shift in the score distributions. 43 uLMS and 578 LM were included in the comparison of pLMS with Zhang's scoring system, with pLMS being superior (AUC 0.960 vs 0.845). LDH, NLR, and PLR achieved a diagnostic odds ratios of 18.03, 8.64 and 4.81, respectively. pLMS2 is based on subscores for menopausal status interacting with age, tumor diameter, intermenstrual bleeding, hypermenorrhea, dysmenorrhea, postmenstrual bleeding, rapid tumor growth, and suspicious sonography.
Conclusions: Validation of the pLMS shows stable discriminatory ability as expressed by AUC, although caution should be taken with cut-off values, as sensitivity and specificity may vary. Data collection of the updated clinical score pLMS2 remains simple and convenient, with no additional cost. The proposed thresholds of 1.5 and 5.5 can be used as a guide to avoid unnecessary or inappropriate surgery and to make the use of further diagnostic measures cost-effective. LDH, NLR and PLR provide further evidence to differentiate uLMS from LM in conjunction with clinical data.
Keywords: Clinical score; Diagnostics; Lactate dehydrogenase; Leiomyoma; Neutrophil-to-lymphocyte-ratio; Platelet-to-lymphocyte-ratio; Uterine leiomyosarcoma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the ethics committee of University Medicine Greifswald (Identifier: BB 034/19a, 2nd March 2021). Written informed consent has been obtained from all patients included in this study for data collection and the use of personal records for research. Consent for publication: Not applicable. Competing interests: Erich-Franz Solomayer declares financial and non-financial competing interests in cooperation with pharmaceutic companies (Amgen, Astra Zeneca, Celgene, Clovis Oncology, Eisai, Erbe, Gedeon Richter, Genomic Health, GSK, Jenapharm, Johnson Johnson, Medac, Mentor, Novartis, Pfizer, Pierre Fabre, Pharma Mar, MSD, Roche, Roche-Posay, Samsung, Storz, Teva, Vifor) and in memberships or leading position in interest groups (German-Romanian Society of the DGGG, German-Hungarian Society of the DGGG, Association of Saarland Surgeons, DGGG, DEGUM (German Society for Ultrasound in Medicine), AGUB (Working Group for Urogynecology of the DGGG), DGS (German Society for Senology), AGO (Working Group for Gynecological Oncology), German Continence Society, DOG (German Society for Osteooncology), German University Association, ARC Gynecology, Saarland Cancer Society, AGE, German Cancer Society, Endometriosis Research Foundation, European Endometriosis League, Association of Friends of the UKS, Working Group for AGCPC, BLFG, Breast Organ Commission of the AGO, AWMF, Saarland Tumor Center at the UKS e.V., UKS/ UdS, ETC, ESGE, FOG, MedConcept, BVF). All other authors declare no competing interests.
Figures
References
-
- Halaska MJ, Haidopoulos D, Guyon F, Morice P, Zapardiel I, Kesic V. European Society of Gynecological Oncology statement on fibroid and uterine morcellation. Int J Gynecol Cancer. 2017;27(1):189–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

