Development of a core outcome set and core measurement set for kangaroo mother care: a study protocol
- PMID: 39773785
- PMCID: PMC11749680
- DOI: 10.1136/bmjopen-2024-089476
Development of a core outcome set and core measurement set for kangaroo mother care: a study protocol
Abstract
Background: Kangaroo mother care (KMC) is a care of preterm and low birthweight infants carried skin-to-skin contact with the mother's chest and breastfeeding when possible. KMC has been proven to reduce mortality and morbidity in these infants. However, research on KMC has been limited by significant variability and inconsistency in reported outcomes across studies. These discrepancies hinder the inclusion of KMC clinical research in systematic reviews or meta-analyses, reducing its research value, leading to resource wastage and raising concerns about selective reporting biases. A core outcome set (COS), which defines a list of critical outcomes, can help harmonise the outcomes reported across studies in the same healthcare field. Further, how these outcomes should be measured and/or reported is defined in a core measurement set (CMS). This study aims to develop both a COS and a CMS for KMC to standardise outcome reporting, improve the quality assessments in clinical trials and facilitate data integration. This protocol outlines the methodology for developing a COS and CMS for KMC.
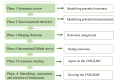
Methods and analysis: The development of the COS and CMS for KMC will follow six phases: (1) a systematic review, (2) semistructured interviews, (3) merging outcomes, (4) two/three rounds of international Delphi survey, (5) a consensus meeting and (6) development of the CMS. In phases 1 and 2, we will conduct a systematic review and semistructured interviews to identify potential core outcomes and measurements, which will form an initial outcome pool. In phase 3, these outcomes will be categorised into domains based on the core outcome measures for effectiveness (COMET) classification, creating a long list of outcomes for the Delphi survey. In phase 4, the Delphi survey will involve two/three rounds with key stakeholders, including neonatal clinical experts (including doctors and nurses), users of COS (including editors, public health experts, experts in evidence-based medicine and researchers), parents of neonates and policymakers, to refine the candidate core outcomes and measurements. In phase 5, an online consensus meeting with representatives of all stakeholders will finalise the COS. In phase 6, the CMS will be following Consensus-based Standards for the selection of health Measurement Instruments guidelines, which involve conceptual considerations, finding existing outcome measurement instruments, assessing their quality and selecting appropriate instruments for the COS. Parents of neonates will participate in phases 2, 4 and 5.
Ethics and dissemination: Ethical approval of this study has been granted by the Medical Ethics Committee of West China Second University Hospital (Medical Research 2024 ethics approval no. 167). The finalised COS and CMS will be disseminated through publication in peer-reviewed journals.
Trial registration number: We have registered the COS in the COMET database (http://www.comet-initiative.org/Studies/Details/2940).
Keywords: NEONATOLOGY; Neonatal intensive & critical care; Nursing Care.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Statistical TC. The China statistical yearbook 2023. [10-May-2024]. https://www.stats.gov.cn/sj/ndsj/2023/indexch.htm Available. Accessed.
MeSH terms
LinkOut - more resources
Full Text Sources