Metabolic fitness of NAC1-deficient Tregs in the tumor microenvironment fuels tumor growth
- PMID: 39773913
- PMCID: PMC11949012
- DOI: 10.1172/jci.insight.186000
Metabolic fitness of NAC1-deficient Tregs in the tumor microenvironment fuels tumor growth
Abstract
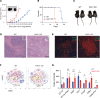
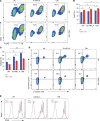
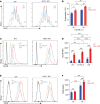
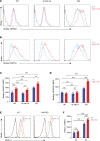
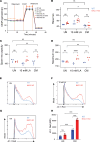
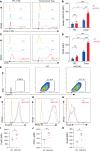
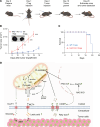
The nucleus accumbens-associated protein 1 (NAC1) has recently emerged as a pivotal factor in oncogenesis by promoting glycolysis. Deletion of NAC1 in regulatory T cells (Tregs) has been shown to enhance FoxP3 stability, a suppressor of glycolysis. This study delves into the intriguing dual role of NAC1, uncovering that Treg-specific deletion of NAC1 fosters metabolic fitness in Tregs, thereby promoting tumorigenesis. Our results unveil that NAC1-deficient Tregs exhibited prolonged survival and heightened function, particularly in acidic environments. Mechanistically, we find that NAC1-deficient Tregs adapted to adverse conditions by upregulating FoxP3 expression, engaging in CD36-mediated lipid metabolism, and enhancing peroxisome proliferator-activated receptor gamma coactivator 1-alpha-regulated mitochondrial function. In mouse tumor xenograft models, NAC1-deficient mice demonstrated increased susceptibility to tumor growth. Notably, Tregs lacking NAC1 not only displayed elevated lipid metabolism and mitochondrial fitness but also exhibited enhanced tumoral infiltration. Adoptive Treg transfer experiments further underscored the supportive role of NAC1-deficient Tregs in tumor growth. These findings suggest that modulating NAC1 expression in FoxP3+ Tregs could serve as a promising approach to augment antitumor immunity. Understanding the intricate interplay between NAC1 and Tregs opens avenues for potential therapeutic strategies targeting the tumor microenvironment.
Keywords: Cancer; Immunology.
Conflict of interest statement
Figures
References
-
- Rahman MT, et al. NAC1, a BTB/POZ protein overexpressed in uterine sarcomas. Anticancer Res. 2012;32(9):3841–3845. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

