Intestinal M icrobiota Transplant Prior to A llogeneic S tem Cell T ransplant (MAST) trial: study protocol for a multicentre, double-blinded, placebo-controlled, phase IIa trial
- PMID: 39773995
- PMCID: PMC11884074
- DOI: 10.1136/bmjopen-2024-093120
Intestinal M icrobiota Transplant Prior to A llogeneic S tem Cell T ransplant (MAST) trial: study protocol for a multicentre, double-blinded, placebo-controlled, phase IIa trial
Abstract
Introduction: Lower diversity of the gut microbiome prior to allogeneic haematopoietic cell transplantation (HCT) correlates with reduced survival after the intervention. Most patients undergoing HCT for a haematological malignancy have previously received intensive chemotherapy, resulting in prolonged neutropenic episodes requiring broad-spectrum antibiotics; use of these has been linked to reduced microbiome diversity. Intestinal microbiota transplant (IMT) is a novel treatment approach that restores this diversity. We hypothesised that IMT performed prior to initiation of HCT conditioning restores microbiome diversity during the early stages of HCT, leading to decreased frequency of complications and improved outcomes of HCT.
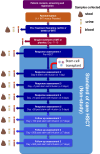
Methods and analysis: 50 adult patients receiving allogeneic HCT will be recruited into this phase IIa trial and randomised 1:1 to receive capsulised IMT or matched placebo shortly prior to initiation of HCT conditioning and followed for up to 12 months. The primary outcome will be to assess the increase in alpha diversity between pre-IMT and that measured at ~42 days after IMT administration (day +28 of HCT), comparing the difference between patients receiving IMT compared with placebo. Secondary outcomes will include tolerability, the dynamics of gut microbiome diversity metrics and taxonomy over all time points assessed, as well as clinical outcomes (including burden of invasive infections, days of fever, admission to intensive care, development of graft-vs-host disease and mortality).
Ethics and dissemination: This study was approved by a UK Research Ethics Committee (REC reference: 23/NE/0105). Dissemination of results will be in concert with patient and public involvement group input and is expected to be primarily via abstract presentation at conferences and manuscripts in peer-reviewed journals.
Trial registration numbers: NCT6355583; EudraCT: 2022-003617-10.
Trial registration: ClinicalTrials.gov NCT06355583.
Keywords: Bone marrow transplantation; Leukaemia; Microbiota; Transplant medicine.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: GW is an employee of and holds share in GSK. All other authors have nothing to report
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
