Mitochondria- and ER-associated actin are required for mitochondrial fusion
- PMID: 39774009
- PMCID: PMC11707194
- DOI: 10.1038/s41467-024-55758-x
Mitochondria- and ER-associated actin are required for mitochondrial fusion
Abstract
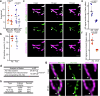
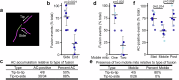
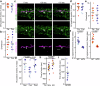
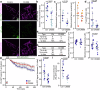
Mitochondria are crucial for cellular metabolism and signalling. Mitochondrial activity is modulated by mitochondrial fission and fusion, which are required to properly balance metabolic functions, transfer material between mitochondria, and remove defective mitochondria. Mitochondrial fission occurs at mitochondria-endoplasmic reticulum (ER) contact sites, and requires the formation of actin filaments that drive mitochondrial constriction and the recruitment of the fission protein DRP1. The role of actin in mitochondrial fusion remains entirely unexplored. Here we show that preventing actin polymerisation on either mitochondria or the ER disrupts both fission and fusion. We show that fusion but not fission is dependent on Arp2/3, whereas both fission and fusion require INF2 formin-dependent actin polymerization. We also show that mitochondria-associated actin marks fusion sites prior to the fusion protein MFN2. Together, our work introduces a method for perturbing organelle-associated actin and demonstrates a previously unknown role for actin in mitochondrial fusion.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Update of
-
Mitochondria- and ER-associated actin are required for mitochondrial fusion.bioRxiv [Preprint]. 2024 Jul 6:2023.06.13.544768. doi: 10.1101/2023.06.13.544768. bioRxiv. 2024. Update in: Nat Commun. 2025 Jan 7;16(1):451. doi: 10.1038/s41467-024-55758-x. PMID: 37398222 Free PMC article. Updated. Preprint.
Similar articles
-
An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2.Science. 2013 Jan 25;339(6118):464-7. doi: 10.1126/science.1228360. Science. 2013. PMID: 23349293 Free PMC article.
-
Mitochondria- and ER-associated actin are required for mitochondrial fusion.bioRxiv [Preprint]. 2024 Jul 6:2023.06.13.544768. doi: 10.1101/2023.06.13.544768. bioRxiv. 2024. Update in: Nat Commun. 2025 Jan 7;16(1):451. doi: 10.1038/s41467-024-55758-x. PMID: 37398222 Free PMC article. Updated. Preprint.
-
INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction, and division.J Cell Biol. 2018 Jan 2;217(1):251-268. doi: 10.1083/jcb.201709111. Epub 2017 Nov 15. J Cell Biol. 2018. PMID: 29142021 Free PMC article.
-
Mitochondrial division, fusion and degradation.J Biochem. 2020 Mar 1;167(3):233-241. doi: 10.1093/jb/mvz106. J Biochem. 2020. PMID: 31800050 Free PMC article. Review.
-
New insights into the function and regulation of mitochondrial fission.Biochim Biophys Acta. 2013 May;1833(5):1256-68. doi: 10.1016/j.bbamcr.2013.02.002. Epub 2013 Feb 20. Biochim Biophys Acta. 2013. PMID: 23434681 Review.
Cited by
-
Endo180 and basement membrane stiffness induce OXPHOS and neoplastic transformation in aging prostate epithelia.NPJ Aging. 2025 Aug 2;11(1):72. doi: 10.1038/s41514-025-00259-4. NPJ Aging. 2025. PMID: 40753084 Free PMC article.
-
Visualizing the internalization and biological impact of nanoplastics in live intestinal organoids by Fluorescence Lifetime Imaging Microscopy (FLIM).Light Sci Appl. 2025 Aug 12;14(1):272. doi: 10.1038/s41377-025-01949-0. Light Sci Appl. 2025. PMID: 40796554 Free PMC article.
-
Representing Mitochondrial Dynamics with Abstract Algebra.bioRxiv [Preprint]. 2025 Mar 28:2025.03.17.643721. doi: 10.1101/2025.03.17.643721. bioRxiv. 2025. PMID: 40166344 Free PMC article. Preprint.
-
The power of connections: Recent advances in understanding the regulation of mitochondrial dynamics by membrane contact sites.Curr Opin Cell Biol. 2025 Aug;95:102535. doi: 10.1016/j.ceb.2025.102535. Epub 2025 May 28. Curr Opin Cell Biol. 2025. PMID: 40441071 Review.
References
MeSH terms
Substances
Grants and funding
- RGPIN-2019-07197/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- F32 GM137580/GM/NIGMS NIH HHS/United States
- S10 OD030505/OD/NIH HHS/United States
- P30 AG068635/AG/NIA NIH HHS/United States
- R01 DC021075/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

