Health-related Quality of Life in Children and Adolescents After Successful Treatment of Chronic Hepatitis C With Sofosbuvir/Velpatasvir: One-year Outcomes
- PMID: 39774052
- PMCID: PMC11980881
- DOI: 10.1097/INF.0000000000004675
Health-related Quality of Life in Children and Adolescents After Successful Treatment of Chronic Hepatitis C With Sofosbuvir/Velpatasvir: One-year Outcomes
Abstract
Background and aims: The aim of this study was to assess the health-related quality of life (HRQL) of children with chronic hepatitis C (CHC) at 1 year after the effective treatment with sofosbuvir/velpatasvir (SOF/VEL).
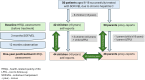
Methods: All 50 patients treated for CHC with a fixed dose SOF/VEL in the noncommercial, nonrandomized, open-label PANDAA-PED study achieved sustained virologic response at 12 weeks after the end of treatment. Evaluation of HRQL at 1-year posttreatment was compared with the baseline (before the treatment) assessment. KIDSCREEN-27 questionnaires, which included 5 dimensions of HRQL, for child self-reporting and parent proxy reporting were used. The normal range for the population was set to T values of 40-60 points. Child-parent agreement was analyzed using the intraclass correlation coefficient (ICC).
Results: Mean T values were within the normal range for all HRQL dimensions. A significant improvement in "autonomy & parent relation" in children's self-assessment (from 48.3 to 51.5, P = 0.03) was observed. In parent proxy assessment, a significant decrease occurred in "school" dimension (from 49.5 to 45.8, P = 0.03), which was not revealed at 3-month posttreatment. Older age was associated with worse HRQL scores in all dimensions. Evaluation of the ICC for child self-reports versus parent proxy reports revealed poor-to-moderate agreement for most single measures, lower than at 3-month posttreatment analysis.
Conclusions: This is the first study to present the long-term influence of treatment with direct-acting antivirals on patient-reported outcomes in children. At 1 year after effective treatment with SOF/VEL, an improvement in some areas of children's well-being was revealed, which may indicate also some patient-reported outcomes benefits of direct-acting antiviral therapy. Despite the improvement in the child self-report of "autonomy & parent relation," there was a more pronounced discrepancy between children self-reports and parents proxy reports in all dimensions of HRQL. Older patients' age correlated with worse HRQL assessment. If this finding is mediated by the duration of hepatitis C virus infection, it would support recommendation for the treatment of younger children.
Keywords: children; health-related quality of life; hepatitis C virus; sofosbuvir/velpatasvir.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

Similar articles
-
Health-related quality of life in patients aged 6-18 years with chronic hepatitis C treated with sofosbuvir/velpatasvir.Liver Int. 2024 Jan;44(1):93-102. doi: 10.1111/liv.15744. Epub 2023 Sep 21. Liver Int. 2024. PMID: 37735963
-
The Influence of Treatment With Sofosbuvir/Velpatasvir on Children's Growth-Results of the PANDAA-PED Study.Pediatr Infect Dis J. 2025 Jan 1;44(1):1-5. doi: 10.1097/INF.0000000000004504. Epub 2024 Sep 4. Pediatr Infect Dis J. 2025. PMID: 39230270 Free PMC article.
-
Real-life effectiveness of sofosbuvir/velpatasvir/voxilaprevir in hepatitis C patients previously treated with sofosbuvir/velpatasvir or glecaprevir/pibrentasvir.Aliment Pharmacol Ther. 2024 Jul;60(2):201-211. doi: 10.1111/apt.18020. Epub 2024 May 2. Aliment Pharmacol Ther. 2024. PMID: 38695095
-
Review article: novel antivirals for hepatitis C-sofosbuvir/velpatasvir/voxilaprevir, glecaprevir/pibrentasvir.Aliment Pharmacol Ther. 2018 Nov;48(9):914-923. doi: 10.1111/apt.14977. Epub 2018 Oct 4. Aliment Pharmacol Ther. 2018. PMID: 30288771 Review.
-
Sofosbuvir plus velpatasvir combination for the treatment of chronic hepatitis C in patients with end stage renal disease on renal replacement therapy: A systematic review and meta-analysis.Nephrology (Carlton). 2022 Jan;27(1):82-89. doi: 10.1111/nep.13968. Epub 2021 Sep 14. Nephrology (Carlton). 2022. PMID: 34453374
References
-
- Younossi Z, Papatheodoridis G, Cacoub P, et al. . The comprehensive outcomes of hepatitis C virus infection: a multi-faceted chronic disease. J Viral Hepat. 2018;25:6–14. - PubMed
-
- Younossi ZM, Stepanova M, Racila A, et al. . Long-term benefits of sustained virologic response for patient-reported outcomes in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2020;18:468–476.e11. - PubMed
-
- Indolfi G, Gonzalez-Peralta RP, Jonas MM, et al. ; Hepatology Committee of the ESPGHAN. ESPGHAN recommendations on treatment of chronic hepatitis C virus infection in adolescents and children including those living in resource-limited settings. J Pediatr Gastroenterol Nutr. 2024;78:957–972. - PubMed
-
- Indolfi G, Easterbrook P, Giometto S, et al. . Efficacy and safety of DAA in children and adolescents with chronic HCV infection: a systematic review and meta-analysis. Liver Int. 2024;44:663–681. - PubMed
-
- Younossi ZM, Stepanova M, Schwarz KB, et al. . Quality of life in adolescents with hepatitis C treated with sofosbuvir and ribavirin. J Viral Hepat. 2018;25:354–362. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

