The WAVE complex in developmental and adulthood brain disorders
- PMID: 39774290
- PMCID: PMC11799376
- DOI: 10.1038/s12276-024-01386-w
The WAVE complex in developmental and adulthood brain disorders
Abstract
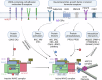
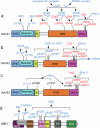
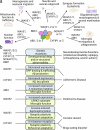
Actin polymerization and depolymerization are fundamental cellular processes required not only for the embryonic and postnatal development of the brain but also for the maintenance of neuronal plasticity and survival in the adult and aging brain. The orchestrated organization of actin filaments is controlled by various actin regulatory proteins. Wiskott‒Aldrich syndrome protein-family verprolin-homologous protein (WAVE) members are key activators of ARP2/3 complex-mediated actin polymerization. WAVE proteins exist as heteropentameric complexes together with regulatory proteins, including CYFIP, NCKAP, ABI and BRK1. The activity of the WAVE complex is tightly regulated by extracellular cues and intracellular signaling to execute its roles in specific intracellular events in brain cells. Notably, dysregulation of the WAVE complex and WAVE complex-mediated cellular processes confers vulnerability to a variety of brain disorders. De novo mutations in WAVE genes and other components of the WAVE complex have been identified in patients with developmental disorders such as intellectual disability, epileptic seizures, schizophrenia, and/or autism spectrum disorder. In addition, alterations in the WAVE complex are implicated in the pathophysiology of Alzheimer's disease and Parkinson's disease, as well as in behavioral adaptations to psychostimulants or maladaptive feeding.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Goley, E. D. & Welch, M. D. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol.7, 713–726 (2006). - PubMed
-
- Krause, M. & Gautreau, A. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol.15, 577–590 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

