Canine Best disease as a translational model
- PMID: 39774293
- PMCID: PMC11794707
- DOI: 10.1038/s41433-024-03578-0
Canine Best disease as a translational model
Abstract
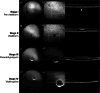
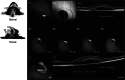
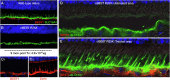
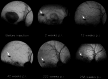
In this review, we summarize the findings of several pre-clinical studies in the canine BEST1 disease model. To this end, client-owned and purpose bred dogs that were compound heterozygotes or homozygotes, respectively, for two or one of 3 different mutations in BEST1 were evaluated by ophthalmic examination, cSLO/sdOCT imaging, and retinal immunohistochemistry to characterize the clinical and microanatomic features of the disease. Subsequently AAV-mediated gene therapy was done to transfer the BEST1 transgene to the RPE under control of a hVMD2 promoter. We demonstrated that canine bestrophinopathies are an RPE-photoreceptor interface disease with underdeveloped RPE apical microvilli that invest rod and cone outer segments. This leads to microdetachments which later progress to clinically evident RPE-retinal separation and a spectrum of disease stages, ranging from vitelliform to vitelliruptive/atrophic lesions, similar to Best Vitelliform Macular Dystrophy (BVMD). Gene therapy corrects the microdetachments and reverses large lesions when delivered at the pseudohypopyon stage of disease. Because of the similar clinical and microstructural abnormalities between the canine model and BVMD, and positive response to gene therapy, the canine model is a valuable translational model for developing gene and other therapies for BVMD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The University of Pennsylvania has pending patent applications for Best Disease gene therapy and the two co-authors are among the co-inventors listed in the patent applications; the technology has been licensed to Opus Genetics, Inc. by the University of Pennsylvania. This work was supported by the Foundation Fighting Blindness Large Animal Model Translational & Research Center grant; NEI/NIH grants EY006855, EY017549; Van Sloun Fund for Canine Genetic Research; and the Sanford and Susan Greenberg End Blindness Outstanding Achievement Prize (GDA).
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

