Cascading expression of ApiAP2 transcription factors controls daughter cell assembly in Toxoplasma gondii
- PMID: 39774584
- PMCID: PMC11723607
- DOI: 10.1371/journal.ppat.1012810
Cascading expression of ApiAP2 transcription factors controls daughter cell assembly in Toxoplasma gondii
Abstract
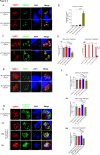
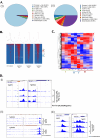
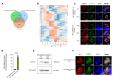
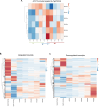
Pathogenesis of Toxoplasma gondii in the intermediate host is based on the tachyzoite ability to divide rapidly to produce significant amount of daughter cells in a reduce time frame. The regulation of the cell-cycle specific expression program is therefore key to their proliferation. Transcriptional regulation has a crucial role in establishing this expression program and transcription factors regulates many aspects of tachyzoite cell cycle. We explored the role of two ApiAP2 transcription factors, TgAP2XII-9 and TgAP2III-2, during the cell cycle of the tachyzoite form. While TgAP2III-2 has only a minor impact on the tachyzoite proliferation, we show that TgAP2XII-9 regulates many aspects of the cell cycle including the proper assembly of the daughter cells inner membrane complex and temporal expression of many virulence genes. Creation of a double mutant strain for TgAP2XII-9 and TgAP2III-2 shows that TgAP2XII-9 had a prominent role during daughter cell assembly. Using transcriptomics and Cut&Tag, we demonstrate that TgAP2XII-9 mainly acts through the transcriptional control of at least 300 genes promoters. Interestingly, TgAP2XII-9 plays a crucial role repressing the expression of genes necessary for budding initiation and activating genes necessary for microneme de novo formation. We also explored the importance of the AP2 domain of TgAP2XII-9 demonstrating its critical role to exert its function. Therefore, we showed that TgAP2XII-9 is a crucial transcription factor which is key to daughter cell assembly post budding initiation.
Copyright: © 2024 Bhaskaran et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

