Aspirin and Hemocompatibility After LVAD Implantation in Patients With Atherosclerotic Vascular Disease: A Secondary Analysis From the ARIES-HM3 Randomized Clinical Trial
- PMID: 39774588
- PMCID: PMC11904737
- DOI: 10.1001/jamacardio.2024.4849
Aspirin and Hemocompatibility After LVAD Implantation in Patients With Atherosclerotic Vascular Disease: A Secondary Analysis From the ARIES-HM3 Randomized Clinical Trial
Erratum in
-
Error in Figure 2.JAMA Cardiol. 2025 Jun 1;10(6):635. doi: 10.1001/jamacardio.2025.1212. JAMA Cardiol. 2025. PMID: 40266587 Free PMC article. No abstract available.
Abstract
Importance: The Aspirin and Hemocompatibility Events With a Left Ventricular Assist Device in Advanced Heart Failure (ARIES-HM3) study demonstrated that aspirin may be safely eliminated from the antithrombotic regimen after HeartMate 3 (HM3 [Abbott Cardiovascular]) left ventricular assist device (LVAD) implantation. This prespecified analysis explored whether conditions requiring aspirin (prior percutaneous coronary intervention [PCI], coronary artery bypass grafting [CABG], stroke, or peripheral vascular disease [PVD]) would influence outcomes differentially with aspirin avoidance.
Objective: To analyze aspirin avoidance on hemocompatibility-related adverse events (HRAEs) at 1 year after implant in patients with a history of CABG, PCI, stroke, or PVD.
Design, setting, and participants: This was an international, multicenter, prospective, double-blind, placebo-controlled, randomized clinical trial including patients implanted with a de novo HM3 LVAD across 51 centers. Data analysis was conducted from April to July 2024.
Interventions: Patients were randomized in a 1:1 ratio to receive aspirin (100 mg per day) or placebo, in addition to a vitamin K antagonist (VKA) targeted to an international normalized ratio of 2 to 3 in both groups.
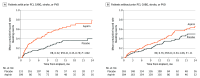
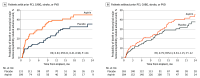
Main outcomes and measures: Primary end point (assessed for noninferiority) was a composite of survival free of any nonsurgical (>14 days after implant) HRAEs including stroke, pump thrombosis, bleeding, and arterial peripheral thromboembolism at 12 months. Secondary end points included nonsurgical bleeding, stroke, and pump thrombosis events.
Results: Among 589 of 628 patients (mean [SD] age, 57.1 [13.7] years; 456 male [77.4%]) who contributed to the primary end point analysis, a history of PCI, CABG, stroke, or PVD was present in 41% (240 of 589 patients). There was no interaction between the presence of an atherosclerotic vascular condition and effect of aspirin compared with placebo (P for interaction= .23). The preset 10% noninferiority margin was not crossed for the studied subgroup of patients. Thrombotic events were rare, with no differences between aspirin and placebo in patients with and without vascular disease (P for interaction = .77). Aspirin treatment was associated with a higher rate of nonsurgical major bleeding events in the group with prior vascular condition history compared with those without aspirin (rate ratio for placebo compared with aspirin, 0.52; 95% CI, 0.35-0.79).
Conclusions and relevance: Results of this prespecified analysis of the ARIES-HM3 randomized clinical trial demonstrate that in patients with advanced heart failure who have classical indications for antiplatelet therapy use at the time of LVAD implantation, aspirin avoidance was safe and not associated with increased thrombosis risk. Importantly, elimination of aspirin was associated with no increased thrombosis but a reduction in nonsurgical bleeding events in patients with a history of PCI, CABG, stroke, or PVD.
Trial registration: ClinicalTrials.gov Identifier: NCT04069156.
Conflict of interest statement
Figures

Comment on
-
Omitting Aspirin in High-Risk Left Ventricular Assist Device Implant.JAMA Cardiol. 2025 Mar 1;10(3):243-244. doi: 10.1001/jamacardio.2024.4861. JAMA Cardiol. 2025. PMID: 39774623 No abstract available.
References
-
- Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364-e467. doi: 10.1161/STR.0000000000000375 - DOI - PubMed
-
- Aboyans V, Ricco JB, Bartelink MEL, et al. ; ESC Scientific Document Group . 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: the European Stroke Organization (ESO)the task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39(9):763-816. doi: 10.1093/eurheartj/ehx095 - DOI - PubMed
-
- Amsterdam EA, Wenger NK, Brindis RG, et al. ; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;130(25):e344-e426. doi: 10.1161/CIR.0000000000000134 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

