Neuro-Behçet's disease: an update of clinical diagnosis, biomarkers, and immunopathogenesis
- PMID: 39774671
- PMCID: PMC11755846
- DOI: 10.1093/cei/uxae123
Neuro-Behçet's disease: an update of clinical diagnosis, biomarkers, and immunopathogenesis
Abstract
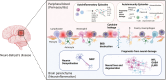
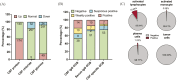
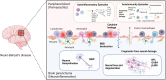
Neuro-Behçet's disease (NBD) is a more severe but rare symptom of Behçet's disease, which is mainly divided into parenchymal NBD (p-NBD) involving brain stem, spinal cord, and cerebral cortex. Non-p-NBD manifests as intracranial aneurysm, cerebral venous thrombosis, peripheral nervous system injuries, and mixed parenchymal and non-parenchymal disease. p-NBD is pathologically characterized by perivasculitis presenting with cerebrospinal fluid (CSF) pleocytosis, elevated total protein, and central nervous system (CNS) infiltration of macrophages and neutrophils, which are subdivided into acute and chronic progressive stages according to relapsing-remitting courses and responses to steroids. The diagnosis of NBD depends heavily on clinical features and magnetic resonance imaging (MRI) findings. The lack of laboratory biomarkers has hindered standard diagnostics. CSF interleukin (IL)-6 is the most investigated dimension of NBD and correlates with NBD activity, therapeutic responses, and prognosis. Further investigations have focused on inflammatory biomarkers that reflect the activation of innate and adaptive immune responses. Higher levels of CSF migration inhibitory factor and immunosuppressive acidic protein indicated the activation of macrophages in the CNS; increased IL-17, IL-10, T-bet/GATA-3, and retinoic acid related orphan receptor (ROR)-γt/Foxp3 ratios, marking the disrupted scale of the Th1/Th2 and Th17/Treg axis; and elevated B-cell activating factor of the TNF family (BAFF) and IgA/IgM intrathecal synthesis, suggesting that B cells play a dominant role in NBD. CNS destruction and degeneration as a consequence of neuroinflammatory cascades were confirmed by elevated CSF levels of NFL, β2MG, and MBP. Autoantibodies, including anti-STIP-1, anti-Mtch1, anti-B-Crystallin, and anti-m-Hsp65, provide substantial evidence for autoimmune essence and underlying microbiological infections in NBD immunopathogenesis. We summarized opinions on the clinical diagnosis, biomarkers, and pathological findings of NBD.
Keywords: biomarkers; blood–brain barrier; cerebrospinal fluid; diagnosis; immunopathogenesis; neuro-Behçet’s disease.
© The Author(s) 2025. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
References
-
- Borhani-Haghighi A, Kardeh B, Banerjee S, Yadollahikhales G, Safari A, Sahraian MA, et al. Neuro-Behcet’s disease: An update on diagnosis, differential diagnoses, and treatment. Mult Scler Relat Disord 2020, 39, 101906. doi: https://doi.org/10.1016/j.msard.2019.101906 - DOI - PubMed
-
- Al-Araji A, Kidd DP.. Neuro-Behçet’s disease: epidemiology, clinical characteristics, and management. Lancet Neurol 2009, 8, 192–204. doi: https://doi.org/10.1016/S1474-4422(09)70015-8 - DOI - PubMed
-
- Akman-Demir G, Serdaroglu P, Tasçi B.. Clinical patterns of neurological involvement in Behçet’s disease: evaluation of 200 patients. The Neuro-Behçet Study Group . Brain 1999, 122 (Pt 11), 2171–82. doi: https://doi.org/10.1093/brain/122.11.2171 - DOI - PubMed
-
- Hirohata S, Kikuchi H, Sawada T, Nagafuchi H, Kuwana M, Takeno M, et al. Clinical characteristics of neuro-Behcet’s disease in Japan: a multicenter retrospective analysis. Mod Rheumatol 2012, 22, 405–13. doi: https://doi.org/10.1007/s10165-011-0533-5 - DOI - PMC - PubMed
-
- Kalra S, Silman A, Akman-Demir G, Bohlega S, Borhani-Haghighi A, Constantinescu CS, et al. Diagnosis and management of neuro-Behçet’s disease: international consensus recommendations. J Neurol 2014, 261, 1662–76. doi: https://doi.org/10.1007/s00415-013-7209-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

