Nematode-bacteria interactions in bovine parasitic otitis
- PMID: 39774744
- PMCID: PMC11756828
- DOI: 10.1590/S1984-29612024081
Nematode-bacteria interactions in bovine parasitic otitis
Abstract
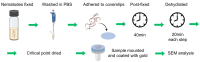
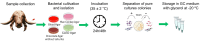
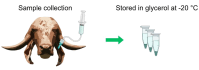
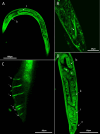
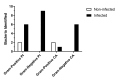
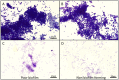
Bovine parasitic otitis poses challenges in diagnosis, treatment and involves various agents, such as bacteria, fungi, mites, and nematodes. This study focused on the nematodes and bacteria isolated from the auditory canals of dairy cattle. A total of twenty samples were collected from dairy cattle in two states of Brazil. The results showed that Metarhabditis freitasi and M. costai nematodes were identified in 75% of samples. Bacterial species from the ear, identified via mass spectrometry, revealed that different strains were present in 65% of the cattle. Mycoplasma spp. were identified in 45% of samples through molecular techniques. Gram-negative bacteria and Mycoplasma spp. were exclusively found in nematode-infected cattle. Furthermore, the bacteria exhibited resistance to multiple antimicrobial classes, and demonstrating multiresistance. Electron microscopy revealed biofilm aggregates on the cuticle of Metarhabditis spp., suggesting a potential role of these nematodes in bacterial migration and interaction with nervous tissue. Thirteen bacterial strains demonstrated biofilm formation ability, indicating their potential pathogenic role. This research highlights the persistent and complex nature of parasitic otitis, emphasizing the significant role of nematode-bacteria associations in its pathogenicity. The presence of resistant strains and biofilm formation underscores the challenges in managing the diagnosis and treatment of bovine parasitic otitis.
A otite parasitária bovina apresenta desafios no diagnóstico e tratamento, envolvendo agentes como bactérias, fungos, ácaros e nematoides. Este estudo focou nos nematoides e bactérias de canais auditivos de gado leiteiro. Um total de vinte amostras foi coletado de bovinos em dois estados do Brasil. Os resultados mostraram nematoides Metarhabditis freitasi e M. costai identificados em 75% das amostras. Espécies bacterianas foram identificadas por espectrometria de massa, revelando a presença de diferentes cepas em 65% do gado. Mycoplasma spp. foram identificados em 45% das amostras usando técnicas moleculares. Gram-negativas e Mycoplasma spp., foram encontradas exclusivamente em bovinos parasitados. Além disso, bactérias exibiram resistência a várias classes de antimicrobianos e demonstraram multirresistência. A microscopia eletrônica revelou agregados de biofilme sobre a cutícula de Metarhabditis spp., sugerindo um possível papel desses nematoides na migração bacteriana e na interação com tecido nervoso. Além disso, treze cepas bacterianas demonstraram capacidade de formação de biofilme, indicando seu potencial patogênico. Este trabalho destaca a natureza persistente e complexa da otite parasitária, enfatizando o papel da associação entre nematoides e bactérias em sua patogenicidade. A presença de cepas resistentes a antimicrobianos e a formação de biofilme, ressalta desafios no manejo do diagnóstico e tratamento da otite parasitária.
Conflict of interest statement
Figures
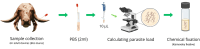

References
-
- Asif M, Prasad JS, Khan R, Somasekhar N, Tahseen Q. A revision of the genus Metarhabditis (Nematoda: Rhabditidae) with description of three known species, a key to the identification of congeners and discussion of their relationships. J Nat Hist. 2013;47(41-42):2599–2622. doi: 10.1080/00222933.2013.798702. - DOI
-
- Atkinson S, Goldstone RJ, Joshua GWP, Chang CY, Patrick HL, Cámara M, et al. Biofilm development on Caenorhabditis elegans by Yersinia is facilitated by quorum sensing-dependent repression of type III secretion. PLoS Pathog. 2011;7(1):e1001250. doi: 10.1371/journal.ppat.1001250. - DOI - PMC - PubMed
-
- Bhat AH, Srivastava S, Rana A, Chaubey AK, Machado RAR, Abolafia J. Morphological, morphometrical, and molecular characterization of Metarhabditis amsactae (Ali, Pervez, Andrabi, Sharma and Verma, 2011) Sudhaus, 2011 (Rhabditida, Rhabditidae) from India and proposal of Metarhabditis longicaudata as a junior synonym of M. amsactae. J Nematol. 2020;52(1):e2020–e2116. doi: 10.21307/jofnem-2020-116. - DOI - PMC - PubMed
-
- Braga PAC, Gonçalves JL, Barreiro JR, Ferreira RC, Tomazi T, Eberlin MN, et al. Rapid identification of bovine mastitis pathogens by MALDI-TOF Mass Spectrometry. Pesq Vet Bras. 2018;38(4):586–594. doi: 10.1590/1678-5150-pvb-4821. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

