Patient-reported improvements in paroxysmal nocturnal hemoglobinuria treated with iptacopan from 2 phase 3 studies
- PMID: 39774762
- PMCID: PMC12008622
- DOI: 10.1182/bloodadvances.2024014652
Patient-reported improvements in paroxysmal nocturnal hemoglobinuria treated with iptacopan from 2 phase 3 studies
Abstract
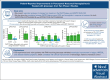
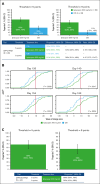
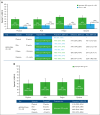
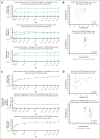
Iptacopan, a first-in-class, oral, selective complement factor B inhibitor, demonstrated efficacy and safety as monotherapy in C5 inhibitor (C5i)-experienced (APPLY-PNH; NCT04558918) and C5i-naive (APPOINT-PNH; NCT04820530) patients with paroxysmal nocturnal hemoglobinuria (PNH). In the APPLY-PNH and APPOINT-PNH trials, changes in fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-Fatigue]) and health-related quality of life (HRQOL; European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire [EORTC QLQ-C30]) from baseline to day 168 were evaluated. The proportion of patients achieving meaningful within-patient change (MWPC) on the FACIT-Fatigue and 4 EORTC QLQ-C30 subscales was evaluated using anchor-based thresholds, and correlations between FACIT-Fatigue scores, lactate dehydrogenase (LDH), and hemoglobin (Hb) levels were assessed. In APPLY-PNH (iptacopan, n = 62; C5i, n = 33), more patients in the iptacopan versus the C5i group reached the MWPC threshold for FACIT-Fatigue (51% vs 11%). More patients achieved MWPC on EORTC QLQ-C30 subscales in the iptacopan group (39%-49%) versus the C5i group (9%-20%). In APPOINT-PNH (N = 40), 56% achieved MWPC on the FACIT-Fatigue, and the proportion of patients who achieved MWPC on the EORTC QLQ-C30 ranged from 41% to 55%. In C5i-experienced patients, increased Hb levels correlated with improvement in FACIT-Fatigue scores (R = 0.48); in C5i-naive patients, increased Hb (R = 0.42) and decreased LDH (R = -0.53) (all P < .001) correlated with improved FACIT-Fatigue scores. C5i-experienced and -naive patients receiving iptacopan exhibited meaningful improvement in fatigue, HRQOL, and disease-related symptoms, which correlated with clinical improvement in hematologic markers of disease control.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.M.R. reports research support from Alexion, Novartis, Alnylam, and Ra Pharma; consultancy from Amyndas; speakers fees from Alexion, Novartis, Pfizer, and Sobi; and membership on an entity’s board of directors or advisory committees of Alexion, Apellis, Novartis, F. Hoffmann-La Roche, Achillion, and Samsung. C.d.C. reports consulting fees from Alexion and Apellis; honoraria from BioCryst, Omeros, and Regeneron; speakers fees from Alexion, Apellis, and Novartis; and membership on an entity’s board of directors or advisory committees of Novartis. A.K. reports research support from Novartis and Bristol Myers Squibb; consulting fees from Alexion, Novartis, Amgen, Agios, Pfizer, Samsung, Celgene, F. Hoffmann-La Roche, and Sobi; honoraria from Alexion, Novartis, Pfizer, Amgen, Samsung, Celgene, F. Hoffmann-La Roche, Bristol Myers Squibb, Sobi, and Silence Therapeutics; and speakers fees from Alexion, Novartis, Amgen, Pfizer, Celgene, F. Hoffmann-La Roche, Sobi, and Ra Pharma. J.P.M. reports consulting fees from Regeneron and Omeros; honoraria from Novartis and Regeneron; speakers fees from Novartis; and membership on an entity’s board of directors or advisory committees of Alexion. P.S. reports research support from Alnylam, BioCryst, Novartis, Pfizer, and F. Hoffmann-La Roche; consulting fees from Alexion, AstraZeneca, BioCryst, Janssen, Novartis, Pfizer, and F. Hoffmann-La Roche; and speakers fees from Alexion, AstraZeneca, Bristol Myers Squibb, Janssen, Novartis, Pfizer, F. Hoffmann-La Roche, and Novartis. Y.U. reports research support from Chugai; consulting fees from Alexion, Asahi Kase, Chugai, Janssen, Novartis, Ono, Sanofi, and Sobi; honoraria from Alexion, Chugai, Kaken, Nippon Shinyaku, Sobi, Incyte, and Sanofi; speakers fees from Alexion, Novartis, and Sanofi; and membership on an entity’s board of directors or advisory committees of Alexion, Janssen, Novartis, and Sanofi. S.V. is an employee of Novartis Services Incorporated and holds shares and/or stock options in the company. G.B. was an employee of Novartis Pharma AG. M.D. is an employee of Novartis Pharma AG and holds shares and/or stock options in the company. R.K. is an employee of Novartis Healthcare Private Limited and holds shares and/or stock options in the company. R.P.d.L. reports research support from Alexion, Pfizer, Novartis, Jazz, and Amgen; consulting fees from Alexion, Pfizer, Novartis, Sobi, Roche, Samsung, Keocyt, Merck Sharp & Dohme, Gilead, Jazz, and Amgen; and honoraria from Alexion, Pfizer, Novartis, Sobi, Roche, Samsung, Keocyt, Merck Sharp & Dohme, Gilead, Jazz, and Amgen. B.H. declares no competing financial interests.
Figures
References
-
- Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121(25):4985–4996. Quiz 5105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

