Genetically engineered pig heart transplantation in non-human primates
- PMID: 39774817
- PMCID: PMC11707197
- DOI: 10.1038/s43856-025-00731-y
Genetically engineered pig heart transplantation in non-human primates
Abstract
Background: Improvement in gene modifications of donor pigs has led to the prevention of early cardiac xenograft rejection and significantly prolonged cardiac xenograft survival in both heterotopic and orthotopic preclinical non-human primate (NHP) models. This progress formed the basis for FDA approval for compassionate use transplants in two patients.
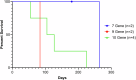
Methods: Based on our earlier report of 9-month survival of seven gene-edited (7-GE) hearts transplanted (life-supporting orthotopic) in baboons, we transplanted 10 gene-edited pig hearts into baboons (n = 4) using non-ischemic continuous perfusion preservation (NICP) and immunosuppression regimen based on co-stimulation blockade by anti-CD40 monoclonal antibody. This pivotal study expands on the 7-GE backbone, with 3 additional gene edits, using 10-GE pigs as donors to baboon recipients.
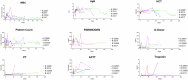
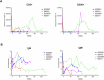
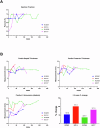
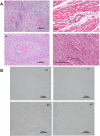
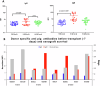
Results: 10 GE cardiac xenografts provide life-supporting function up to 225 days (mean 128 ± 36 days) in a non-human primate model. Undetectable or latent porcine cytomegalovirus (PCMV) does not influence cardiac xenograft survival in this study but still needs more exploration with a larger cohort. Xenograft histology demonstrates adipose (Fat) deposition (n = 1), chronic vasculopathy (n = 1), micro and macro thrombosis, and acute cellular rejection (n = 1).
Conclusions: These data demonstrate that 10 GE cardiac xenografts have variable cardiac xenograft survival in NHP due to perhaps presence of 4th antigen and require further study. However, these 10GE organs may be suitable for clinical cardiac xenotransplantation and have already been utilized in two human cases.
Plain language summary
There is a shortage of organs donated for use in transplantation. Instead, animal organs could potentially be used for people with end-stage organ failure. We modified pig hearts to make them more like human organs and transplanted them into non-human primates. The pig hearts functioned in the non-human primates for up to 225 days. These hearts could also potentially be used in people with heart failure.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: All other authors except K.K. and D.A. declare no competing interests or personal relationships that could have appeared to influence the work reported in this article. K.K. and D.A. are employees of Revivicor Inc., Blacksburg, VA, USA, and got a salary, as part of the data acquisition for this study but their role could not have appeared to influence the work reported in this article.
Figures

References
-
- Neergarard, L. Surgeons Perform Second Pig Heart Transplant, Trying to Save a Dying Man (Associated Press, 2023).
-
- Mohiuddin, M. et al. The second GE pig heart transplant in a living human offers great hope for xenotransplantation. In Amertican Transplnat Congress (ed. Transplantation AJo) (2024).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

