Prevalence of urinary tract infections in women with vulvovaginal atrophy and the impact of vaginal prasterone on the rate of urinary tract infections
- PMID: 39774900
- PMCID: PMC12147743
- DOI: 10.1097/GME.0000000000002485
Prevalence of urinary tract infections in women with vulvovaginal atrophy and the impact of vaginal prasterone on the rate of urinary tract infections
Abstract
Objective: The aims of this study were to assess the prevalence of urinary tract infections (UTI) in women newly diagnosed with vulvovaginal atrophy (VVA) versus women without VVA and to evaluate the potential of vaginal prasterone to be used in postmenopausal VVA women with UTI as prophylaxis to reduce the future UTI risk. As a first subgroup analysis, women using aromatase inhibitors, medications that stop the production of estrogen were analyzed. As a second subgroup analysis, we looked at women with diabetes to investigate whether the same prophylaxis approach should be considered.
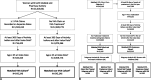
Methods: This observational retrospective inception cohort study was conducted using the Integrated Dataverse open-source claims database with data from February 2015 through January 2020.
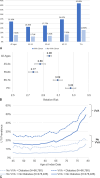
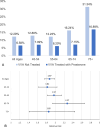
Results: A total of 22,245 women treated with prasterone for a minimum of 12 weeks were matched to women without any prescribed VVA-related treatment. Overall, women treated with prasterone have a significantly lower UTI prevalence compared to those untreated (6.58% vs 12.3%; P < 0.0001). The highest difference in UTI prevalence among the prasterone treated and untreated women was observed in those aged 65-74 (7.15% vs 16.2%; P < 0.0001). Among aromatase inhibitor users and women with diabetes, those treated with prasterone have a significantly lower UTI prevalence (4.90% vs 9.79%; P < 0.01 and 14.59% vs 20.48%; P < 0.0001, respectively).
Conclusions: This study suggests that intravaginal prasterone may be a good candidate for prophylaxis in postmenopausal women with UTI to reduce future UTI risk, including for women taking aromatase inhibitors and women with diabetes. This study is based on real-world evidence and warrants further investigation in a clinical setting.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The Menopause Society.
Conflict of interest statement
Financial disclosure/conflicts of interest: E.M. and K.D. are consultants for Endoceutics. A.D. is an employee of Cosette Pharmaceuticals, Inc. M.S. has received funding from Lupin Pharma Ltd for unrelated work. A.Y. receives ongoing institutional funding from Sexual Medicine Society of North America and past funding from International Society for the Study of Women's Sexual Health and Clarus Therapeutics. The other authors have nothing to disclose.
Figures
References
-
- Portman DJ Gass ML, Vulvovaginal Atrophy Terminology Consensus Conference Panel . Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause 2014;21:1063–1068. 10.1097/GME.0000000000000329 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

