Survival benefit associated with liver transplantation for hepatocellular carcinoma based on tumor burden scores at listing
- PMID: 39774957
- PMCID: PMC11717502
- DOI: 10.1097/HC9.0000000000000619
Survival benefit associated with liver transplantation for hepatocellular carcinoma based on tumor burden scores at listing
Abstract
Introduction: Liver transplantation (LT) provides significant survival benefits to patients with unresectable HCC. In the United States, organ allocation policies for HCCs within the United Network for Organ Sharing criteria do not prioritize patients based on their differences in oncological characteristics. This study assessed whether transplant-associated survival benefits (TASBs) vary among patients with different tumor burden scores (TBS) measured at the time of listing.
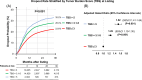
Methods: We analyzed data from adults applying for HCC MELD exception points between 2002 and 2019, with follow-up until December 2023, using the Scientific Registry of Transplant Recipients. TBS was determined based on the largest tumor diameter and number of HCCs. Patients were categorized into low (≤3), intermediate (3.1-5), and high (>5) TBS groups. TASB was measured as the difference in 5-year survival with and without LT.
Results: This study included 36,634 LT candidates. High-TBS patients had higher waitlist dropout rates and marginally lower post-transplant survival, resulting in a significantly greater TASB. The 5-year TASB for the low, intermediate, and high TBS groups were 15.7, 22.1, and 25.0 months, respectively. The adjusted survival benefit expressed in 5-year survival differences was 21.9%, 34.5%, and 39.4% in the low, intermediate, and high TBS groups, respectively (p<0.001).
Conclusions: Higher TBS during listing correlates with greater LT benefits for patients with unresectable HCC within UNOS criteria. We conclude that organ allocation policies in the United States should prioritize patients with high TBS due to their increased risk of dropout and comparable post-transplant survival when compared to patients with less advanced tumors.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Mengyang Di is on the speaker’s bureau for Intellisphere LLC. She received grants from Schrodinger and DAVA Oncology. Ramon Bataller consults for Novo Nordisk, GSK, Boehringer Ingelheim, and Resolution Therapeutics. He is on the speaker’s bureau for Gilead and Abbvie. Jaideep Behari received grants from Pfizer, Gilead, and AstraZeneca. Andres Duarte-Rojo received grants from Echosens. The remaining authors have no conflicts to report.
Figures



References
-
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. - PubMed
-
- Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. - PubMed
-
- Shimamura T, Akamatsu N, Fujiyoshi M, Kawaguchi A, Morita S, Kawasaki S, et al. Expanded living-donor liver transplantation criteria for patients with hepatocellular carcinoma based on the Japanese nationwide survey: The 5-5-500 rule—A retrospective study. Transpl Int. 2019;32:356–368. - PubMed
-
- Kwon CH, Kim DJ, Han YS, Park JB, Choi GS, Kim SJ, et al. HCC in living donor liver transplantation: Can we expand the Milan criteria? Dig Dis. 2007;25:313–319. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

